Key Points
-
Four human CD1 proteins — CD1A, CD1B, CD1C and CD1D — present lipid antigens by insertion of lipids into a groove in the proteins to form CD1–lipid complexes. These complexes activate T cells after direct recognition by specific T-cell receptors.
-
Crystal structures have been determined for two CD1 proteins. These show that the mouse CD1d antigen-binding groove has two pockets, which can accommodate lipids with an overall length of up to ∼C40, whereas the human CD1B groove is composed of four pockets and can bind larger lipid antigens.
-
CD1B-mediated presentation of long-chain lipids (C54–80) generally requires that CD1B and antigens be exposed to low pH, such as that found in late endosomal compartments.
-
Adaptor-protein complexes (AP1, AP2 and AP3) regulate the intracellular trafficking of CD1 proteins by physically binding to tyrosine-based and modified dileucine motifs in the cytoplasmic tails of CD1 proteins.
-
After synthesis in the endoplasmic reticulum, CD1A, CD1B, CD1C and CD1D traverse the secretory pathway to the cell surface. The cytoplasmic tails of each of these CD1 isoforms interact differently with adaptor-protein complexes, leading to distinct patterns of steady-state distribution in subcompartments of the endosomal network.
-
The cytoplasmic tail sequences of CD1 proteins control their intracellular trafficking and their ability to select and activate CD1-restricted T cells. This indicates that normal patterns of intracellular trafficking of CD1 proteins regulate T-cell activation, in part by altering the spectrum of lipid antigens that are bound in the grooves of CD1 molecules.
-
Low-efficiency loading of lipids in non-endosomal compartments provides a mechanism for presenting self-lipids on an ongoing basis. High-efficiency presentation of lipids through endosomal pathways allows cells to sample exogenously acquired antigens, including those from microbial pathogens.
Abstract
Each of the human CD1 proteins takes a different route through secretory and endocytic compartments before finally arriving at the cell surface, where these proteins present glycolipid antigens to T cells. Recent studies have shown that adaptor-protein complexes and CD1-associated chaperones control not only CD1 trafficking, but also the development and activation of CD1-restricted T cells. This indicates that CD1 proteins, similar to MHC class I and II molecules, selectively acquire certain antigens in distinct cellular subcompartments. Here, we summarize evidence supporting the hypothesis that CD1 proteins use separate, but parallel, pathways to survey endosomal compartments differentially for lipid antigens.
Similar content being viewed by others
Main
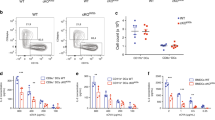
For many years, it was thought that peptides were the only structurally varied targets of T-cell responses. The discovery of CD1-dependent antigen-presentation pathways provided a mechanism by which T cells can specifically recognize an array of lipids and glycolipids that comprise the membranes of mammalian cells and microbial pathogens1. Mouse CD1d, and guinea-pig CD1b and CD1c, as well as four of the five human CD1 proteins (CD1A, CD1B, CD1C and CD1D), have been shown to bind and present lipid antigens to T cells2,3,4,5,6,7. Similar to MHC class I and II molecules, CD1 proteins present antigens by loading one of many possible antigenic compounds into a groove on the distal surface of the protein, forming a stable antigen complex that is recognized directly by T-cell receptors (TCRs). X-ray crystallographic studies have shown that CD1d and CD1B proteins have large antigen-binding grooves that are lined by hydrophobic amino acids, which interact with the alkyl chains of amphipathic lipids8,9. This mechanism of binding anchors the alkyl chains in the CD1 groove, so that the naturally variable carbohydrate, or other hydrophilic components of the antigen, protrude from the groove, making them available for direct interaction with antigen-specific TCRs (Fig. 1).
Human CD1B–lipid complexes were prepared from denatured CD1B heavy chains refolded in the presence of phosphatidylinositol (shown) or GM2 ganglioside, and detergent9. The heavy chain (blue) is composed of three extracellular domains (α1, α2 and α3), which associate non-covalently with β2-microglobulin (β2-m; light green). The α1 and α2 domains of CD1B form an antigen-binding groove that is substantially larger than that found in mouse CD1d8. The CD1B groove is composed of four pockets — A′, C′, F′ and T′ — which were named after the corresponding A′ and F′ pockets in mouse CD1d, the C′ pocket in MHC class I molecules and a 'tunnel,' which is unique to CD1B. The inositol ring (dark green) protrudes from the groove and lies on the α-helical surface of CD1B in the predicted region of T-cell receptor binding, on the basis of mutational studies and computer models 18,87. The two alkyl chains (C16 and C18) of phosphatidylinositol lie in the A′ (red) and C′ (yellow) pockets, respectively, and two C16 molecules lie in the T′ tunnel (violet) and the F′ pocket (pink). Reprinted, with permission, from Ref. 9 © (2002) Macmillan Magazines Ltd.
Several classes of lipid antigen are presented by CD1 molecules, including mycobacterial mycolates, phosphatidylinositols, sphingolipids and polyisoprenoid lipids10,11,12,13,14,15. These known antigens, together with diffferentially glycosylated derivatives of these lipids that might function as antigens, form a potentially large pool of structures that could be recognized by CD1-restricted T cells. Although certain CD1D-restricted natural killer T (NKT) cells use TCRs of limited diversity, the available evidence indicates that the CD1-restricted T-cell repertoire also includes T cells with substantial TCR diversity16,17,18,19,20. Functional studies of T-cell fine specificity for antigen structure have shown that CD1-restricted T-cell clones can discriminate between CD1 isoforms, as well as the precise structures of lipids that are bound in the CD1 groove2,13,21. So, the CD1 system mediates highly specific T-cell responses to various lipid antigens.
It is now clear that many lipid antigens do not simply bind CD1 at the cell surface, but instead are recognized by T cells after undergoing processing or loading in intracellular compartments4,22,23,24,25,26,27,28,29. The different cellular requirements for presenting various classes of lipid antigen raise the possibility that CD1 proteins bind different types of antigen as they pass through secretory, cell-surface or endosomal pathways, much as MHC class I and II molecules survey cytosolic and endosomal compartments, respectively, for peptide antigens. Here, we summarize how adaptor-protein complexes and CD1-associated chaperones control the intracellular trafficking of CD1 proteins, focusing on how intracellular sorting events affect the subsequent activation of lipid-specific T cells. Two separate, but parallel, pathways can be identified — one that requires endosomal co-factors for antigen presentation and one that does not. We speculate that use of the highly efficient endosomal pathway promotes the presentation of foreign glycolipids that are internalized from exogenous sources into antigen-presenting cells (APCs). By contrast, the more abundant self-lipids that comprise the membranes of APCs can be presented to autoreactive T cells using less efficient loading mechanisms that are present in various non-endosomal compartments.
Translation and assembly of CD1 proteins
The human CD1 locus maps outside the MHC and encodes five CD1 proteins, known as CD1A, CD1B, CD1C, CD1D and CD1E30. The polypeptides that are encoded by the CD1 genes contain three extracellular domains (α1, α2 and α3) and are known as heavy chains because of their homology to MHC class I heavy chains1 (Fig. 1). Similar to MHC class I heavy chains, the CD1 heavy chains form heterodimeric complexes in the endoplasmic reticulum (ER) that consist of one heavy chain non-covalently paired with one β2-microglobulin (β2-m) light-chain subunit. All CD1 protein sequences contain a leader peptide, which signals co-translational insertion of the heavy chain into the ER membrane, such that the α1 and α2 domains, which form the antigen-binding pocket of CD1, and the α3 domain are in the lumen of the ER. This leaves the short tails of the CD1 heavy chains, which are composed generally of 6–10 amino acids, protruding into the cytoplasmic compartment. Alternative messenger-RNA splice variants that could potentially encode secreted proteins have been described for CD1A, CD1C and CD1E31,32. Although CD1E proteins are expressed intracellularly, it is not known yet whether they are expressed on the cell surface also or are involved in T-cell activation32. So, further discussion of CD1 function in this article focuses on the other four human isoforms and their homologues in other mammals, which are known to present lipid antigens to T cells.
As shown for CD1B, shortly after translation into the ER, CD1 heavy chains associate rapidly with the protein-folding chaperones calnexin and calreticulin33,34. Blockade of these interactions by glucosidase inhibitors prevents the efficient egress of CD1B heavy chains to the cell surface, which indicates that these interactions are important for normal CD1 heavy-chain folding34. Folding of the CD1 heavy chains brings the hydrophobic amino acids of the α1 and α2 domains into close proximity, so that they form a nearly continuous hydrophobic surface that constitutes the inner surface of the CD1 antigen-binding groove8,9. It has not been established conclusively whether CD1 normally folds around ER-resident lipids. Although it has been possible to refold bacterially synthesized CD1 heavy chains with β2-m in the absence of added lipids using an oxidative refolding system, other findings indicate that the refolding of denatured CD1B molecules in the presence of β2-m can be facilitated by the addition of certain detergents, which seem to function as lipid chaperones by becoming incorporated into the hydrophobic binding groove9,35,36. In addition, CD1D proteins produced in Drosophila cells have been found to have a bound ligand containing two hydrocarbon chains in the antigen-binding groove when crystallized (I. Wilson, personal communication). This indicates that these secreted CD1 proteins are loaded naturally with lipids, and that these lipids are not oriented randomly in the groove, because they give rise to a well-defined electron-density map when bound in the groove.
In addition to promoting the generation of a hydrophobic surface in aqueous solution, chaperone lipids loaded onto CD1 molecules in the ER have been proposed to have a physiological role in blocking the CD1 groove, analogous to the known function of class II invariant chain (Ii) peptide (CLIP) in binding to MHC class II molecules. For CD1D, phosphatidylinositol-containing (PI) glycolipids have been proposed to have such a role, as they have been detected as the predominant lipids in eluates of cellular CD1D proteins. In addition, functional studies indicate that the CD1D–PI association might occur in the ER, or at least in the secretory pathway37,38. The addition of exogenous mammalian PI to APCs in vitro can lead to the activation of CD1D-restricted T-cell hybridomas, so certain forms of PI could function as antigens that are recognized by T cells39. More generally, it is probable that ubiquitous self-ligands, such as PI, that are bound to CD1 molecules at early stages in the secretory pathway function as non-antigenic lipid chaperones that protect the CD1 groove before the CD1 molecules encounter more-antigenic lipids during trafficking through later stages of the secretory or endocytic compartments (Fig. 2). A more comprehensive understanding of the identity and affinity of self-ligands that bind to CD1 molecules in the ER could be important for understanding how these ligands regulate the exchange for exogenously derived antigenic lipids at later stages in the antigen-processing pathway.
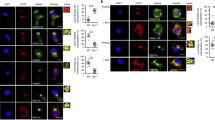
Human CD1 isoforms differ in their steady-state distribution, as shown by merged confocal images of CD1-transfected HeLa cells stained with isoform-specific anti-CD1 antibodies (green) and anti-LAMP1 antibody (red), a marker of lysosomes46. Co-localization of CD1 with LAMP1 is shown in yellow. After export by the secretory pathway, CD1A is found mainly at the cell surface and in clathrin-coated pits and sorting endosomes (se). The cytoplasmic tails of CD1C and CD1D contain tyrosine-based (YXXZ; where Y is tyrosine, X is any amino acid and Z is a bulky hydrophobic residue) motifs, which bind to adaptor protein-2 (AP2) complexes at the cell surface. This interaction mediates sorting into clathrin-coated vesicles and delivery to intermediate and late endosomes (le). The tails of human, but not mouse, CD1D proteins also contain a functional modified dileucine motif (not shown), which acts as a second signal to mediate the sorting of human CD1D to late endosomes. In addition, a fraction of CD1D proteins that associate with MHC class-II–invariant-chain complexes are diverted to late endosomes or MHC class II compartments (MIIC). This diversion to late endosomes is likely to occur on the basis of the interaction of modified dileucine motifs (LL or IL) in the tails of invariant chain (dark pink) and MHC class II molecules (light pink). The YXXZ motif of CD1B is unique among the human CD1 isoforms in its ability to bind AP3, which promotes sorting to lysosomal compartments and MIIC. Recent studies have shown that AP3 also influences the intracellular localization of mouse CD1d. ee, early endosome; ER, endoplasmic reticulum; LAMP1, lysosome-associated membrane protein 1; TGN, trans-Golgi network. Confocal images are reprinted, with permission from Elsevier Science © (2002), from Ref. 46.
A second event of CD1 assembly in the ER involves the non-covalent association of CD1 heavy chains with β2-m. All five of the human CD1 isoforms can associate with β2-m, but they do so with varying affinity. CD1B–β2-m interactions are particularly strong, and they are resistant to dissolution at a pH as low as 3.0. So, CD1B might be particularly well suited to functioning in the low-pH environment of late endosomes and lysosomes29. Most of the evidence indicates that the normal cell-surface expression of CD1 proteins requires association with β2-m. In fact, association with β2-m might function to regulate the egress of CD1 proteins from the ER, as it does for MHC class I molecules. In support of this, the association of β2-m with CD1B heavy chains occurs almost concurrently with the acquisition of ENDOGLYCOSIDASE-H RESISTANCE, and the expression of CD1A, CD1B or CD1C heavy chains in cells lacking β2-m leads to their rapid degradation34,40,41. The functional importance of the association of β2-m with CD1D has been shown in studies of β2-m-deficient mice, which have impaired development of CD1d-restricted NKT cells and markedly reduced efficiency of CD1-restricted T-cell activation42.
Exit to the surface and recycling
Each of the human CD1 heavy chains has multiple N-linked glycosylation sites, and the heavy chains are heavily glycosylated during transport through the Golgi apparatus, a process that facilitates measurement of the kinetics of CD1 transport through the secretory pathway. As discussed in detail later, a subpopulation of CD1D proteins are bound up in MHC class-II–Ii complexes and are thought to be delivered directly to late endosomal compartments from the trans-Golgi network43,44. However, large amounts of newly synthesized CD1D and CD1B proteins are detected at the cell surface within one hour of acquiring endoglycosidase-H resistance44,45. This rapid transit time, which is comparable to that of MHC class I molecules, indicates that most CD1B and CD1D proteins transit first to the cell surface before reaching endosomes. The cell surface-to-endosome pathway has been shown to exist by labelling CD1B or CD1D proteins at the cell surface and then measuring rates of re-internalization directly. These experiments show that more than half of the pool of cell-surface CD1B and CD1D molecules is re-internalized within one hour, and for CD1D, there is evidence for many rounds of recycling between the cell surface and endosomes44,45.
The rapid rate of re-internalization provides further evidence that the main route of trafficking of CD1B and CD1D to endosomal compartments involves a prior stop at the cell surface, an indirect pathway that is distinct from that taken by MHC class-II–Ii complexes (Fig. 2). As many studies indicate that reaching endosomes is crucially important for normal glycolipid-antigen processing, this raises the question of why CD1 proteins take such an indirect route to this compartment4,22,23,24,25,26,27,28. New studies show that isoform-specific sequences in the cytoplasmic tails of CD1 proteins differentially interact with adaptor-protein complexes at the cytoplasmic face of the plasma membrane, allowing the various CD1 isoforms to be sorted from one another and delivered to only partially overlapping subcompartments of the endosomal network45,46. Therefore, the initial trafficking of CD1 proteins to the cell surface might be thought of as positioning each of the human CD1 isoforms for sorting events that govern whether they are delivered selectively to sorting endosomes, early endosomes, late endosomes or lysosomes (Fig. 2).
Control of intracellular localization by adaptors
Adaptor-protein complexes (known as AP1, AP2, AP3 and AP4) are heterotetramers that consist of two large subunits (γ, α, δ or ε paired with β1, β2, β3 or β4), one medium subunit (μ1–μ4) and one small subunit (σ1–σ4)47,48,49 (Fig. 3). Adaptor-protein complexes control intracellular sorting by binding amino-acid motifs in the cytoplasmic tails of certain transmembrane proteins, leading to their packaging into transport vesicles (Table 1). Both tyrosine- and leucine-based cytoplasmic-tail motifs are involved in control of the intracellular sorting of CD1 proteins. A sequence of four amino acids (YXXZ; where Y is tyrosine, X is any amino acid and Z is a bulky hydrophobic residue) mediates binding to the μ-subunit of adaptor-protein heterotetramers (Fig. 3). Mutational studies have shown that both the tyrosine and hydrophobic amino acid are important for this interaction, and the physical basis for this is the insertion of these amino-acid side-chains into conserved pockets in the μ-chain of an adaptor-protein complex50,51. Basic and acidic amino acids in or adjacent to the motif affect sorting by altering the affinity of the YXXZ motif for different μ-subunits, but a comprehensive understanding of the rules that govern affinity has not been achieved yet52.
Adaptor-protein (AP) complexes consist of two large subunits (α, γ, δ or εpaired with β1, β2, β3 or β4), one medium subunit (μ1–4) and one small subunit (σ1–4). They are expressed on the cytoplasmic face of cellular membranes, where they physically interact with the cytoplasmic tails of transmembrane cargo proteins, including CD1B, CD1C and CD1D. The cytoplasmic tails of proteins with YXXZ motifs (where Y is tyrosine, X is any amino acid and Z is a bulky hydrophobic residue) bind AP complexes by insertion of the tyrosine and hydrophobic amino acids into two adjacent pockets in the μ-subunit. AP1 is expressed predominantly at the trans-Golgi network (TGN) and promotes sorting of proteins to the endosomal system, including MHC class-II–invariant-chain complexes. AP2 is expressed at the cell surface and mediates the internalization of CD1B, CD1C and CD1D into clathrin-coated vesicles that can be delivered subsequently to endosomal compartments. AP3 is expressed at the TGN and in endosomes, and it functions in the delivery of CD1B to late endosomes and lysosomes. AP4 complexes have not been shown to have a role in CD1 trafficking. β2-m, β2-microglobulin.
A second kind of cytoplasmic-tail motif that controls CD1 trafficking is the modified dileucine motif. Typically, this sequence contains two adjacent leucine, valine or isoleucine residues, but it might also be comprised of other amino acids53,54. The physical basis of binding of dileucine signals in the tails of cargo proteins to adaptor-protein complexes is less well understood than the binding of tyrosine motifs. There is evidence that modified dileucine motifs bind to the β1- and β2-subunits, and also to the μ1- and μ2-subunits, of AP1 and AP2 (Refs 55–57). Generally, these interactions promote the delivery of transmembrane proteins from the trans-Golgi network to late endosomal or lysosomal compartments, and they have been implicated in the transport of human CD1D, MHC class II molecules and invariant chain to these compartments58,59,60,61.
Sampling of early endosomes by CD1A
CD1A has a particularly short cytoplasmic tail that lacks any known motif that could mediate binding to adaptor proteins and sorting into specialized endosomal compartments (Fig. 2). Consistent with this, electron-microscopy analysis has shown that CD1A is expressed prominently at the cell surface, and immunofluoresence-microscopy studies have shown that CD1A is not expressed at a significant level in lysosome-associated membrane protein 1 (LAMP1)+ late endosomes (Fig. 2). However, CD1A is present in perinuclear, transferrin-receptor-expressing compartments, in clathrin-coated vesicles and in specialized sorting endosomes of Langerhans cells known as Birbeck granules24,46. At present, it is not known whether the entry of CD1A into these intracellular compartments is simply the result of bulk flow of a fraction of cell-surface molecules into CLATHRIN-COATED PITS and early endocytic vesicles, or whether some as-yet-unidentified, specific targeting signal is involved.
The functions of CD1A include the activation of autoreactive T cells, a process that probably includes the presentation of self-antigens11,62. In addition, CD1A presents exogenously acquired polar lipids from mycobacterial cell walls for recognition by antigen-specific T cells6. Studies of the cellular requirements for presentation of these mycobacterial lipid antigens have shown that T-cell activation can be inhibited completely by fixing APCs with glutaraldehyde, but is not blocked by treatment of APCs with concanamycin B, which is a specific inhibitor of vacuolar ATPases24. This indicates that presentation of these antigens requires internalization of CD1A or the antigen into intracellular compartments, but does not require the low pH that is found in late endosomal or lysosomal compartments. The time that is required for processing of these glycolipids by CD1A is also consistent with this conclusion, because kinetic studies show that detectable antigenic complexes are formed after ∼20 minutes. This delay is longer than that observed for antigens that are loaded on the surface, but is shorter than that observed for antigens that require delivery to late endosomes or lysosomes for loading onto CD1 molecules26. So, the intracellular localization, pH requirements and kinetics of antigen presentation all point to a function for CD1A in binding and presenting antigens in the earliest compartments of the endocytic network.
Sampling of early and late endosomes by CD1C
CD1C penetrates further than CD1A into the endosomal network, where it co-localizes with transferrin receptors in early endosomes and can also show limited co-localization with LAMP1, a marker of lysosomes25,63. Recent evidence from SURFACE PLASMON-RESONANCE binding assays and YEAST TWO-HYBRID analyses shows that peptides containing the YXXZ motif that is present in the cytoplasmic tail of CD1C bind to AP2, but not AP3 (Refs 45,46) (Fig. 3). This interaction has a functional role in the sorting of CD1C to endosomes, as deletion of the CD1C tail, which contains the AP2-binding motif, results in the redistribution of CD1C from endosomes to the cell surface under steady-state conditions63.
CD1C functions both to activate autoreactive T cells and to present microbial MANNOSYL PHOSPHOISOPRENOID (MPI) antigens to T cells14,64. Some data indicate that presentation of MPI antigens might require internalization into APCs, because membrane fixatives block the presentation of these antigens25. However, endosome-acidification inhibitors have only a mild effect on antigen presentation, and tail-deleted CD1C proteins can still present MPI antigens to T cells25,63. Overall, these findings indicate that the presentation of an exogenous MPI antigen can occur efficiently at the cell surface but is probably enhanced by delivery to endosomes with an intermediate pH. As the phosphate-ester bonds of MPI antigens can be hydrolysed at the low pH that is typical of lysosomes, it is possible that the delivery of MPI antigens to early endocytic compartments might facilitate antigen presentation, whereas delivery to late endosomes or lysosomes could result in antigen destruction14,65.
Three signals for endosomal localization of CD1D
The cytoplasmic tails of mouse and human CD1D contain a tyrosine motif that is predicted to bind AP2, an interaction that probably controls the sorting of CD1D to late endosomes and the basolateral surface of polarized cells23,28,44,52,66,67 (Fig. 3). Also, recent studies have shown that the trafficking of mouse CD1d to late endosomes is impaired in AP3-deficient cells, which indicates that mouse CD1d might interact with AP3 (M. Cernades, M. Brenner and M. Kronenberg, personal communications). In addition, the human CD1D tail contains a dileucine signal that promotes trafficking to lysosomes when the tyrosine motif is inactivated by mutagenesis66,68. As mouse CD1d lacks this modified dileucine motif, this second endosome-localization signal in human CD1D represents a possible functional difference between these two orthologous proteins (Table 1).
The delivery of CD1D proteins to the endosomal network by tail-encoded sequences is important for their antigen-presenting function, because cells that express tail-deleted CD1D proteins fail to activate NKT cells expressing invariant TCR α-chains (Vα14–Jα18 in mice and Vα24–Jα15 in humans)7,23. In addition, transgenic mice expressing tail-deleted CD1d have a marked reduction in the positive selection of INVARIANT NKT CELLS27. These effects probably result from the failure of tail-deleted CD1D to traffic through the low-pH environment of endosomal compartments, because separate studies have shown that treatment of APCs with endosome-acidification inhibitors produces similar effects on the activation of invariant NKT cells28.
A third mechanism for the transport of CD1D to late endosomal compartments involves the non-covalent association of CD1D with MHC class II–Ii complexes. Mouse and human CD1D proteins have been detected at substoichiometric levels in immunoprecipitates prepared using antibodies specific for invariant chain or for MHC class II molecules43,44. In experiments carried out in cells with tail-deleted CD1D proteins, the expression of invariant chain by transfection or induction of expression of MHC class II molecules promotes the trafficking of CD1D to late endosomes28,43,44. Although the route by which CD1D–MHC-class-II–Ii complexes reach late endosomes has not been established directly, it might involve targeting by modified dileucine motifs in the tails of Ii or the MHC class II β-chain55,59,60,61 (Table 1 and Fig. 2).
Taken together, these data indicate three separate mechanisms by which CD1D can be sorted for delivery to late endosomal compartments: the tyrosine-based motif in the tail of CD1D; a modified dileucine motif in the tail of human CD1D; and modified dileucine motifs in non-covalently associated MHC class II molecules or invariant chain (Table 1 and Fig. 2). However, these three mechanisms are not equivalent in their effects on the presentation of endogenous antigens to T cells. In vitro, the re-routing of tail-deleted CD1D proteins to late endosomes by MHC class-II–Ii complexes can lead to the increased activation of invariant Vα14+ NKT cells44. However, the effects of MHC class II molecules and invariant chain on CD1D trafficking and antigen presentation have been documented only for mutant CD1D proteins that lack the tyrosine and modified dileucine motifs in their tails, a situation that is not encountered in vivo. Moreover, transgenic mice expressing tail-deleted CD1d have a markedly reduced number of invariant NKT cells in vivo, a profound defect that is not rescued by the normal expression of MHC class-II–Ii complexes27. This indicates strongly that the tail-encoded sequences are of greater functional importance than the interaction with MHC class-II–Ii complexes. However, these three mechanisms for endosomal delivery of CD1D are unlikely to represent simply functional redundancy. Studies of MHC class II molecules are providing important insights into functional subcompartments in the traditional late endosomal, lysosomal and MHC class II compartments69,70. Therefore, it is tempting to speculate that these three mechanisms might function normally to deliver CD1D to different, as-yet-unidentified subcompartments of late endosomes and lysosomes, which could have distinct roles in the loading of lipids onto CD1D.
Endosomal proteases and CD1D. Possible insights into the endosomal mechanisms by which CD1D-mediated antigen presentation could be regulated have come from the unexpected finding that deficiency of the cysteine proteases cathepsin S and cathepsin L reduces the development and activation of invariant NKT cells71,72. In one study, cathepsin-S-deficient mice were found to have markedly reduced levels of invariant NKT cells, as determined by staining with α-GALACTOSYL CERAMIDE-loaded CD1d tetramers71. In addition, cathepsin-S-deficient cells showed reduced presentation to NKT cells of a digalactosyl ceramide, an antigen for which recognition is known to depend on endosomal processing71. In other studies, cathepsin-L-deficient mice have been shown to have a nearly complete loss of NKT cells in the periphery, an effect that is stronger than that seen with cathepsin-S deficiency72. In both studies, cathepsin deficiency had effects on NKT-cell development without markedly altering the level of cell-surface expression of CD1d, which indicates that cathepsins might have a functional role in antigen processing or the modification of CD1D proteins.
The molecular mechanism by which the deletion of an endopeptidase could impair lipid-antigen presentation is not known yet. However, cathepsin L is expressed normally by thymic epithelial cells, from which it is secreted and taken up by CD1D-expressing thymocytes72. Therefore, it is found in the correct intracellular compartment to function in the endosomal pathway for presenting endogenous antigens to Vα14+ NKT cells. Cysteine proteases have a known role in cleavage of the invariant chain and the release of MHC class II molecules from endosomes to the cell surface, so they can affect either of these proteins, which physically associate with CD1D43,44,73. Although these and other important questions relating to the molecular events that underlie endosomal antigen processing by CD1D remain unanswered, these studies provide strong functional evidence that the trafficking of CD1D through endosomes regulates the development and activation of invariant Vα14+ NKT cells.
A non-endosomal pathway for antigen presentation. Trafficking of CD1D through endosomes is not required for the activation of all CD1D-restricted T cells. This was shown in experiments in which cells expressing tail-deleted CD1d were as effective as those expressing wild-type CD1d at stimulating mouse T-cell hybridomas or at positively selecting CD1d-restricted T cells that lack Vα14 (for example, Vα3.2–Vβ8+ T cells) and express a more varied TCR repertoire23,27. These different requirements for CD1d trafficking for the activation of Vα14+ and Vα14− T-cell populations have been interpreted to indicate that these two T-cell populations recognize different antigens, and that loading of antigens for presentation to Vα14+ NKT cells occurs in endosomes, whereas loading of antigens for presentation to Vα14− T cells does not. This is a probable explanation, and the identification of such endogenous antigens remains a priority. However, it is also possible that the different patterns of reactivity result from other modifications of CD1D or CD1-presented antigens in endosomes, such as deglycosylation, oligomerization or pH-induced conformational changes.
CD1B trafficking
Human CD1B was the first CD1 protein shown to present lipid antigens, and it is the isoform for which the most information regarding antigen structure exists at present4 (Fig. 4). CD1B binds or presents at least three classes of antigen: mycolates (free MYCOLIC ACID and glucose monomycolate), diacylglycerols (phosphatidylinositol mannoside, lipoarabinomannan and phosphatidylinositol) and sphingolipids (GM1 ganglioside, GM2 ganglioside and sulphatides)3,10,11,12,13,15. Emerging evidence indicates that some of these antigens are presented by mechanisms that require trafficking of CD1B through endosomes, whereas other antigens do not require endosomal processing. These observations, together with recent insights into the molecular features of antigens that control their loading onto CD1B, now point to the existence of parallel, but functionally separate, endosomal and non-endosomal pathways for lipid-antigen presentation by CD1B.
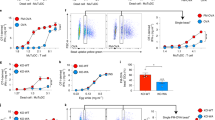
The human CD1B groove has two portals for ligand entry and is composed of four pockets. CD1B can bind phosphatidylinositol, ganglioside GM2 or, possibly, other ligands by inserting approximately 36 methylene units (dashed line) in the A′ and C′ pockets9. Longer lipids, having up to approximately 76 methylene units (solid line), could be accommodated in all four pockets. CD1B presents antigens that vary greatly in chain length, including mycolates, glucose monomycolates (GMMs), gangliosides (GM1), sulphatides, phosphatidylinositol mannosides (PIMs) and liporarabinomannan (LAM)11,12,13,15 Among these, those that have short alkyl chains (<C40) can be loaded rapidly onto cell-surface CD1B proteins or onto cell-free CD1B proteins. CD1B-mediated presentation of antigens with long alkyl chains (>C40) can be inhibited by removing endosome-localization motifs from the CD1B tail or by treating antigen-presenting cells (APCs) with fixatives or endosome-acidification inhibitors. This indicates that endosomal factors selectively promote the presentation of mycobacterial antigens by inserting the longer lipid tails into three or more pockets of the CD1B groove. The size of each known antigen is shown as CX, where X is the total number of methylene units in the lipid portion of each antigen, not taking into account any changes that could occur during processing by APCs. PIM/LAM is shown with two mannosyl residues, although the naturally occurring forms of this glycolipid are much larger owing to the presence of additional mannosyl or arabinosyl residues12.
Surveillance of late endosomes and lysosomes. Of the CD1 isoforms, CD1B seems to penetrate furthest into the late compartments of the endosomal network, efficiently reaching late endosomes, lysosomes and MHC class II compartments22,46,74. Binding studies using surface plasmon-resonance and yeast two-hybrid systems have shown that the tyrosine-based motif YQNIP in the CD1B cytoplasmic tail mediates binding of both AP2 and AP3 (Refs 45,46). This points to a two-step model for the transport of CD1B to lysosomes (Fig. 2). CD1B probably associates with AP2 at the cell surface, which leads to internalization into endosomes, where it binds AP3, resulting in delivery to lysosomes. Consistent with this model, CD1B is more extensively localized than CD1A or CD1C in organelles that co-express LAMP1 (Refs 24,63) (Fig. 2).
The available evidence from in vitro studies indicates that many antigens that are presented by CD1B absolutely require endosomal processing or loading before their recognition by T cells. For example, treatment of APCs with membrane fixatives before antigen exposure prevents the presentation of mycolic acid, glucose monomycolate with long alkyl chains (C80 GMM) and lipoarabinomannan (LAM) to CD1B-restricted T cells4,12,75. Also, these studies showed that treatment of APCs with chloroquine or concanamycin B inhibits T-cell recognition, which implicates a requirement for low pH as the factor that makes endosomal localization necessary for the presentation of these antigens. In addition, low pH facilitates the physical interaction of CD1B with the antigens that it presents, as plasmon-resonance studies have shown that CD1B binds LAM and GMM at pH 4–5, but not at pH 7.4 (Ref. 29). Finally, inhibition of CD1B trafficking to endosomes — by deletion of the CD1B tail, by alanine substitution of the tyrosine residue in the YXXZ motif or by glycosyl-phosphatidylinositol re-anchoring — leads to reduced efficiency of presentation of mycolic acid and C80 GMM to T cells22,76. Taken together, these studies provide strong evidence that the entry of CD1B into the low-pH environment of late endosomes or lysosomes is important for its ability to present these bacterial antigens to T cells.
A non-endosomal pathway for antigen presentation. More recent studies, looking at the presentation of mammalian glycosphingolipid antigens, have questioned whether CD1B trafficking through endosomes is required universally for CD1B-mediated antigen presentation. These studies found that treatment of APCs with membrane fixatives or endosome-acidification inhibitors did not block the presentation of mammalian gangliosides or sulphatides11,15. Moreover, these antigens could be loaded onto recombinant CD1B proteins at neutral pH to form complexes that activate T cells, providing direct evidence against an absolute requirement for any type of endosomal co-factor77. So, certain CD1B-presented glycolipids require processing in endosomal compartments, whereas gangliosides and sulphatides do not. This raises the important question of which molecular features of CD1B-presented antigens determine the requirement for endosomal processing.
Although all of the CD1B-presented antigens are composed of two alkyl chains with a central hydrophilic group, they differ markedly in terms of the overall length of their combined alkane chains (Fig. 4). Sphingolipids and diacylglycerols, which are the most abundant lipid components of mammalian cells, typically have a total of 32 to 46 methylene units in their combined sphingosine base and acyl chain (C32–46)11,15. By contrast, mycobacterial mycolic acids and GMMs are much longer, typically C76–86 (Refs 10,13). Correlation of the overall lipid length with endosomal-processing requirements shows that those antigens with longer alkyl chains typically require endosomal processing for presentation, whereas those with shorter alkyl chains typically do not (Fig. 4). The only known exception to this rule is LAM — an antigen with short lipid chains but containing an unusually large (∼20 kDa) glycan structure — which is delivered directly to late endosomal compartments after binding to the mannose receptor12,78.
This correlation indicates that endosomal processing might be required for the presentation of those antigens that have long alkyl chains, an hypothesis that has been tested recently by comparing the cellular processing requirements of natural and synthetic GMM antigens that ranged incrementally in length from C12 to C80 (Ref. 26). This study showed that lipids with a combined alkyl chain length in the range of C12–32 could be presented rapidly at the surface of fixed cells, whereas T-cell recognition of antigens with the same TCR epitope, but with longer alkyl chains (C54–80), was inhibited by CD1B tail deletion or by treating APCs with fixatives or endosome-acidification inhibitors. Interestingly, cells expressing tail-deleted CD1B proteins presented short-chain antigens with much greater efficiency than did cells expressing high levels of full-length CD1B proteins, which indicates that the passage of CD1B through endosomes inhibits the presentation of short-chain antigens by non-endosomal pathways. These studies indicate that CD1B-expressing APCs can use functionally separate endosomal and non-endosomal pathways for the presentation of antigens and that antigens with larger lipid moieties are presented by the endosomal pathways. The recent solution of the crystal structure of human CD1B now provides insights into the possible molecular mechanisms that might underlie these different processing requirements9.
CD1 antigen-binding grooves
The antigen-binding groove of mouse CD1d contains two connecting pockets, A′ and F′, which together are large enough to accommodate lipids having an overall size of 32–40 methylene units, depending on the position of glycolipids in the groove8. Corresponding to this, the sphingolipid and diacylglycerol antigens that are presented by this isoform have an overall alkyl chain length in this range2,39,79. By contrast, the human CD1B antigen-binding groove is much larger and consists of four adjacent pockets — A′, C′, F′ and the T′ 'tunnel'9 (Fig. 1). For CD1B, there are two 'entrances' from the outer surface of the protein into the pocket — a large opening between the α-helices, which is also present in CD1d and MHC molecules, and a small portal in the lateral wall of the C′ pocket. Therefore, it is possible that the alkyl chains of larger lipids could protrude from the groove through this C′ portal or other sites.
The crystal structure of CD1B provides important insights into how this non-polymorphic antigen-presenting molecule can bind such structurally diverse lipids, including those that range in length from C12 to C80 (Ref. 26) Crystallized, refolded CD1B proteins simultaneously bind three lipids — either GM2 or PI, together with two C16 lipids (Fig. 1). This modular architecture indicates that CD1B could bind diacylglycerol, sphingolipid or other small antigens with an overall alkyl chain length of up to ∼C40 by inserting them directly into the A′ and C′ pockets, as seen in the crystal structure. In this case, the areas of the CD1B antigen-binding groove that are not involved in antigen binding could be occupied by one or more immunologically inert, groove-stabilizing lipids.
Antigens with alkyl chains in the range of C40–76 might occupy three or all four pockets. The loading of yet larger lipids, such as C80 GMM or free mycolic acid, is predicted to require insertion of the lipid moiety into all four pockets, and the lipid might also protrude slightly through the portal in the C′ pocket. This process might require conformational changes to CD1B so that the bound lipid can make contact with all four pockets simultaneously. In addition, insertion of long-chain lipids might involve the expulsion of more than one chaperone lipid (Fig. 1).
This, or other related aspects of antigen loading, could explain why only antigens with lipid-chain lengths in the range of C54–80 require endosomal presentation (Fig. 4). The low pH of late endosomes could promote relaxation of the α-helices, which form the roof and sides of the groove, thereby facilitating access to the groove. Alternatively, endosomes could provide exchange proteins with functions that are analogous to those of HLA-DM in the peptide loading of MHC class II molecules. A third possibility is that endosomal lipases could cleave C80 mycolates so that shorter alkyl chains could fit more easily in four or fewer pockets. As endosomal pathways seem to be more efficient than non-endosomal pathways for the loading of antigens onto CD1B, further investigation of the molecular mechanisms that govern this process, including the role of lipid cleavage or effects of pH on CD1B folding, will be important.
These observations indicate that the trafficking of CD1B to late endosomes and lysosmes could be a specialized mechanism for selectively presenting lipids with long chain lengths, which naturally accumulate in these compartments26,80. As mycobacterial mycolates have longer alkyl chains than self-sphingolipids or -diacylglycerols, the efficient presentation of long-chain antigens in endosomes might even be a mechanism to skew the T-cell response towards lipids that have a more intrinsically foreign structure26 (Fig. 4). This hypothesis can be tested more directly by analysing the chain lengths of lipids that are sorted to endosomes and complexed with CD1B. In addition, it is now possible to begin to determine whether long- or short-chain antigens activate T cells more efficiently in vivo using lipid-loaded tetramers and related techniques to measure the frequencies of lipid-antigen-specific T-cell precursors in infected humans.
Importance of separate processing pathways
Viewed broadly, these studies of the cellular requirements for lipid-antigen presentation by CD1 molecules provide evidence of functionally separate endosomal and non-endosomal pathways for glycolipid-antigen presentation to T cells. For CD1D, the existence of endosomal and non-endosomal pathways could allow APCs to control separately the activation of NKT-cell populations with invariant (Vα14+) and diverse (Vα14−) TCRs23,27. However, clearly defined differences between the normal functions of these two cell populations have not been determined yet, and the structures of the natural endogenous antigens that are presented to them are not known. It will be necessary to resolve these questions to propose an integrated model of how the apparently separate pathways of CD1D antigen processing could regulate immune responses in vivo.
By contrast, there is much information relating the biological origin of microbial antigens, their precise molecular structures and the role of these structures in antigen processing and T-cell activation (Fig. 4). The studies that are reviewed here point to an emerging picture of how the endosomal and non-endosomal CD1-mediated antigen-presentation pathways could control physiological immune responses to foreign and self-glycolipids. We speculate that CD1A, CD1B and CD1C present exogenously acquired foreign antigens to T cells that function in host defence against infection. Intracellular pathogens, including mycobacteria, can inhibit endosomal maturation81,82. Certain antigens, such as mycobacterial mycolates, can accumulate preferentially in lysosomes, whereas others, such as polyisoprenyl phosphates, might be degraded in the low pH of this compartment26,65. For these and other reasons, surveillance for pathogens might be optimized by the fact that CD1A, CD1B and CD1C are specialized to sample different parts of the endosomal network individually, but function together to sample the entire endosomal pathway for pathogens.
Even in the presence of an intracellular infection, microbial lipids form only a small proportion of the total lipids comprising the membranes of APCs. In addition, it is probable that self-lipids are loaded onto CD1 proteins in the ER, before CD1 is exposed to foreign antigens in the endosomal network37,38. Therefore, T-cell activation by foreign lipids during host defence probably requires that cells have mechanisms for removing self-lipids from the CD1 groove and selectively loading bacterial glycolipids onto CD1, analogous to known mechanisms for loading foreign peptides onto endosomal MHC class II proteins73,83. In contrast to cytosolic, ER, secretory and cell-surface compartments, endosomes are likely to be enriched for foreign lipids, because this is the first compartment to acquire live intracellular bacteria or lipid components shed from extracellular pathogens84. In addition, as certain PATTERN-RECOGNITION RECEPTORS can bind foreign lipids specifically, microbial lipids can be internalized selectively for delivery to endosomes78. Therefore, the ability of CD1A, CD1B and CD1C to load bacterial antigens selectively in endosomes could skew the repertoire of lipids that are presented by these molecules towards those of exogenous or foreign origin. This might be particularly true for CD1B, which most clearly requires a low pH for binding lipids and seems to have specialized mechanisms for preferentially presenting long-chain mycobacterial lipids4,26,29.
At the same time, CD1 molecules can also bind and present self-diacylglycerols and -sphingolipids, and many examples of CD1-restricted autoreactive T cells are known. This indicates the existence of cellular pathways for the loading and presentation of endogenous self-lipids. Most studies of the loading of self-lipids onto CD1 molecules show that this occurs readily at the cell surface or, using recombinant CD1 proteins, in neutral biological buffers11,37,39,77 (Fig. 4). This indicates that cellular pathways for presenting self-antigens involve low-stringency loading mechanisms that occur in most subcellular compartments, rather than only the specialized low-pH environment of endosomes. Although the non-endosomal presentation mechanisms are more rapid and less stringent than endosomal mechanisms, in most studies so far, they have been shown to be less efficient. Exogenously administered gangliosides and sulphatides require micromolar concentrations to activate T cells, in contrast to endosomally presented microbial antigens, which can be recognized at low nanomolar concentrations10,11,13,14,15 So, the non-endosomal pathway might function to sample, on a more global scale, abundant self-glycolipids, a role that could be well adapted for T cells that function in immunosurveillance or immunoregulation. Thereby, endosomal and non-endosomal pathways could both carry out important, but separate, functions in an immune response.
Concluding remarks
A more complete understanding of these separate pathways of lipid-antigen processing will involve precisely defining the cellular subcompartments in which lipid antigens and CD1 proteins intersect and the detailed molecular basis for insertion of lipids into the CD1 groove. So far, most studies have focused on the dynamic trafficking patterns of CD1 proteins, rather than those of lipids. Nevertheless, it is clear that certain classes of CD1-presented lipid have a non-random distribution in cellular subcompartments. For example, certain polyisoprenoid lipids accumulate in the ER, whereas sphingolipid glycosylation can occur selectively in the Golgi apparatus. Diacylglycerols with long alkyl chains can be sorted selectively to lysosomes80,85,86. Cellular activation or apoptosis leads to the redistribution of anionic phospholipids to the outer leaflet of the cytoplasmic membrane, and microbial lipids are delivered selectively to endosomes by pattern-recognition receptors present on the surface of maturing dendritic cells78. A clearer understanding of how these intracellular patterns of trafficking and accumulation of lipids lead to loading onto CD1 proteins should provide new insights into the normal biological functions of lipid-specific T cells.
References
Porcelli, S. A. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59, 1–98 (1995).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). Synthetic α-galactosyl ceramides were discovered as the first known antigens for invariant NKT cells. Fine-specificity studies showed that the α-anomeric linkage of the carbohydrate, a modification that is found rarely in mammalian ceramides, is crucial for T-cell activation.
Hiromatsu, K. et al. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J. Immunol. 169, 330–339 (2002).
Porcelli, S., Morita, C. T. & Brenner, M. B. CD1b restricts the response of human CD4−8− T lymphoyctes to a microbial antigen. Nature 360, 593–597 (1992).
Beckman, E. M. et al. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 157, 2795–2803 (1996).
Rosat, J. P. et al. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ αβ T-cell pool. J. Immunol. 162, 366–371 (1999).
Spada, F. M., Koezuka, Y. & Porcelli, S. A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188, 1529–1534 (1998).
Zeng, Z. et al. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277, 339–345 (1997). The first crystal structure of a CD1 protein shows that mouse CD1d has a large hydrophobic antigen-binding groove that is composed of two pockets, A′ and F′.
Gadola, S. D. et al. Structure of human CD1b with bound ligands at 2.3 Å, a maze for alkyl chains. Nature Immunol. 3, 721–726 (2002). This study reports two crystal structures of refolded CD1B proteins bound to the ganglioside GM 2 or phosphatidylinositol. These structures show that the aliphatic hydrocarbon chains of lipids are inserted into the CD1 groove. The human CD1B groove was shown to be larger than that of mouse CD1d, and it is composed of four pockets — A′, C′, F′ and T′. This modular structure shows how CD1B can bind lipids that vary in overall chain length by inserting the lipids into two or more pockets in the groove.
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ T cells. Nature 372, 691–694 (1994).
Shamshiev, A. et al. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195, 1013–1021 (2002).
Sieling, P. A. et al. CD1-restricted T-cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Moody, D. B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997).
Moody, D. B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999). This study provides the first clear functional evidence for the presentation of self-glycolipids by non-endosomal mechanisms.
Koseki, H. et al. Homogenous junctional sequence of the Vα14+ T-cell antigen receptor α-chain expanded in unprimed mice. Proc. Natl Acad. Sci. USA 87, 5248–5252 (1990).
Porcelli, S., Gerdes, D., Fertig, A. M. & Balk, S. P. Human T cells expressing an invariant Vα24–JαQ TCRα are CD4− and heterogeneous with respect to TCRβ expression. Hum. Immunol. 48, 63–67 (1996).
Grant, E. P. et al. Molecular recognition of lipid antigens by T-cell receptors. J. Exp. Med. 189, 195–205 (1999).
Behar, S. M., Podrebarac, T. A., Roy, C. J., Wang, C. R. & Brenner, M. B. Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 (1999).
Cardell, S. et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182, 993–1004 (1995).
Burdin, N. et al. Structural requirements for antigen presentation by mouse CD1. Proc. Natl Acad. Sci. USA 97, 10156–10161 (2000).
Jackman, R. M. et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351 (1998).
Chiu, Y. H. et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189, 103–110 (1999).
Sugita, M. et al. Separate pathways for antigen presentation by CD1 molecules. Immunity 11, 743–752 (1999).
Sugita, M., van Der, W., Rogers, R. A., Peters, P. J. & Brenner, M. B. CD1c molecules broadly survey the endocytic system. Proc. Natl Acad. Sci. 97, 8445–8450 (2000).
Moody, D. B. et al. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nature Immunol. 3, 435–442 (2002). This study provides direct evidence for the role of alkyl chain length in controlling whether lipids are presented by endosomal or non-endosomal pathways.
Chiu, Y. H. et al. Multiple defects in antigen presentation and T-cell development by mice expressing cytoplasmic-tail-truncated CD1d. Nature Immunol. 3, 55–60 (2002).
Roberts, T. J. et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J. Immunol. 168, 5409–5414 (2002).
Ernst, W. A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 (1998).
Calabi, F. & Milstein, C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature 323, 540–543 (1986).
Woolfson, A. & Milstein, C. Alternative splicing generates secretory isoforms of human CD1. Proc. Natl Acad. Sci. USA 91, 6683–6687 (1994).
Angenieux, C. et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J. Biol. Chem. 275, 37757–37764 (2000).
Sugita, M., Porcelli, S. A. & Brenner, M. B. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J. Immunol. 159, 2358–2365 (1997).
Huttinger, R., Staffler, G., Majdic, O. & Stockinger, H. Analysis of the early biogenesis of CD1b: involvement of the chaperones calnexin and calreticulin, the proteasome and β2-microglobulin. Int. Immunol. 11, 1615–1623 (1999).
Karadimitris, A. et al. Human CD1d–glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl Acad. Sci. USA 98, 3294–3298 (2001).
Altamirano, M. M., Blackburn, J. M., Aguayo, C. & Fersht, A. R. Ligand-independent assembly of recombinant human CD1 by using oxidative refolding chromatography. Proc. Natl Acad. Sci. USA 98, 3288–3293 (2001).
Joyce, S. et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279, 1541–1544 (1998).
De Silva, A. D. et al. Lipid–protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J. Immunol. 168, 723–733 (2002).
Gumperz, J. et al. Murine CD1d-restricted T-cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Sugita, M. & Brenner, M. B. An unstable β2-microglobulin: major histocompatibility complex class I heavy chain intermediate dissociates from calnexin and then is stabilized by binding peptide. J. Exp. Med. 180, 2163–2171 (1994).
Bauer, A. et al. Analysis of the requirement for β2-microglobulin for expression and formation of human CD1 antigens. Eur. J. Immunol. 27, 1366–1373 (1997).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995).
Kang, S. J. & Cresswell, P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 21, 1650–1660 (2002). This paper provides evidence for the association of CD1D with MHC class II molecules and for a functional role for MHC class-II–invariant-chain complexes in control of the intracellular trafficking of CD1D.
Jayawardena-Wolf, J., Benlagha, K., Chiu, Y. H., Mehr, R. & Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 15, 897–908 (2001). This study provides evidence for two routes of trafficking of CD1D proteins to late endosomes. CD1D proteins can recycle rapidly from the cell surface to endosomes or they can be delivered to this compartment after association with MHC class-II–invariant-chain complexes.
Briken, V., Jackman, R. M., Dasgupta, S., Hoening, S. & Porcelli, S. A. Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J. 21, 825–834 (2002). These experiments provide evidence for the physical association of the cytoplasmic tail of CD1B with AP2 and AP3 using surface plasmon-resonance assays. In addition, this report provides evidence for the role of this interaction in the intracellular localization of and antigen-presenting function of CD1B.
Sugita, M. et al. Failure of trafficking and antigen presentation by CD1 in AP3-deficient cells. Immunity 16, 697–706 (2002). Using a yeast two-hybrid system, this report provides evidence for the association of CD1B with AP2 and AP3. Human cells deficient in AP3 are shown to have a reduced efficiency of lipid-antigen presentation.
Boehm, M. & Bonifacino, J. S. Genetic analyses of adaptin function from yeast to mammals. Gene 286, 175–186 (2002).
Kantheti, P. et al. Mutation in AP3δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes and synaptic vesicles. Neuron 21, 111–122 (1998).
Dell'Angelica, E. C., Shotelersuk, V., Aguilar, R. C., Gahl, W. A. & Bonifacino, J. S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP3 adaptor. Mol. Cell 3, 11–21 (1999).
Owen, D. J. & Evans, P. R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332 (1998).
Ohno, H. et al. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872–1875 (1995).
Bonifacino, J. S. & Dell'Angelica, E. C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145, 923–926 (1999).
Johnson, K. F. & Kornfeld, S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J. Biol. Chem. 267, 17110–17115 (1992).
Letourneur, F. & Klausner, R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell 69, 1143–1157 (1992).
Rapoport, I., Chen, Y. C., Cupers, P., Shoelson, S. E. & Kirchhausen, T. Dileucine-based sorting signals bind to the β-chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 17, 2148–2155 (1998).
Rodionov, D. G. & Bakke, O. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J. Biol. Chem. 273, 6005–6008 (1998).
Greenberg, M., DeTulleo, L., Rapoport, I., Skowronski, J. & Kirchhausen, T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8, 1239–1242 (1998).
Salamero, J., Le Borgne, R., Saudrais, C., Goud, B. & Hoflack, B. Expression of major histocompatibility complex class II molecules in HeLa cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on Golgi membranes. J. Biol. Chem. 271, 30318–30321 (1996).
Kang, S., Liang, L., Parker, C. D. & Collawn, J. F. Structural requirements for major histocompatibility complex class II invariant chain endocytosis and lysosomal targeting. J. Biol. Chem. 273, 20644–20652 (1998).
Zhong, G., Romagnoli, P. & Germain, R. N. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J. Exp. Med. 185, 429–438 (1997).
Kongsvik, T. L., Honing, S., Bakke, O. & Rodionov, D. G. Mechanism of interaction between leucine-based sorting signals from the invariant chain and clathrin-associated adaptor protein complexes AP1 and AP2. J. Biol. Chem. 277, 16484–16488 (2002).
Porcelli, S. et al. Recognition of cluster of differentiation 1 antigens by human CD4−CD8− cytolytic T lymphocytes. Nature 341, 447–450 (1989).
Briken, V., Jackman, R. M., Watts, G. F., Rogers, R. A. & Porcelli, S. A. Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J. Exp. Med. 192, 281–288 (2000).
Sieling, P. A. et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J. Immunol. 165, 5338–5344 (2000).
Moody, D. B. Polyisoprenyl glycolipids as targets of CD1-mediated T-cell responses. Cell Mol. Life Sci. 58, 1461–1474 (2001).
Rodionov, D. G., Nordeng, T. W., Pedersen, K., Balk, S. P. & Bakke, O. A critical tyrosine residue in the cytoplasmic tail is important for CD1d internalization but not for its basolateral sorting in MDCK cells. J. Immunol. 162, 1488–1495 (1999).
Blumberg, R. S., Colgan, S. P. & Balk, S. P. CD1d: outside-in antigen presentation in the intestinal epithelium? Clin. Exp. Immunol. 109, 223–225 (1997).
Rodionov, D. G., Nordeng, T. W., Kongsvik, T. L. & Bakke, O. The cytoplasmic tail of CD1d contains two overlapping basolateral sorting signals. J. Biol. Chem. 275, 8279–8282 (2000).
Mellman, I. & Steinman, R. M. Dendritic cells: specialized and regulated antigen-processing machines. Cell 106, 255–258 (2001).
Boes, M. et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418, 983–988 (2002).
Riese, R. J. et al. Regulation of CD1 function and NK1.1+ T-cell selection and maturation by cathepsin S. Immunity 15, 909–919 (2001).
Honey, K. et al. Thymocyte expression of cathepsin L is essential for NKT-cell development. Nature Immunol. 3, 1069–1074 (2002).
Nakagawa, T. Y. & Rudensky, A. Y. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol. Rev. 172, 121–129 (1999).
Sugita, M. et al. Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science 273, 349–352 (1996). This report provides the first evidence that tyrosine-based sequences of CD1 proteins have a role in the delivery of CD1 proteins to late compartments in the endosomal network, including the MHC class II compartment.
Moody, D. B., Reinhold, B. B., Reinhold, V. N., Besra, G. S. & Porcelli, S. A. Uptake and processing of glycosylated mycolates for presentation to CD1b-restricted T cells. Immunol. Lett. 65, 85–91 (1999).
Geho, D. H. et al. Glycosyl-phosphatidylinositol reanchoring unmasks distinct antigen-presenting pathways for CD1b and CD1c. J. Immunol. 165, 1272–1277 (2000).
Shamshiev, A. et al. The αβ T-cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity 13, 255–264 (2000).
Prigozy, T. I. et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6, 187–197 (1997).
Brossay, L. et al. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J. Immunol. 161, 5124–5128 (1998).
Mukherjee, S., Soe, T. T. & Maxfield, F. R. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 144, 1271–1284 (1999).
Ferrari, G., Langen, H., Naito, M. & Pieters, J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97, 435–447 (1999).
Pancholi, P., Mirza, A., Bhardwaj, N. & Steinman, R. M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260, 984–986 (1993).
Cresswell, P. Invariant chain structure and MHC class II function. Cell 84, 505–507 (1996).
Schaible, U. E., Hagens, K., Fischer, K., Collins, H. L. & Kaufmann, S. H. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 164, 4843–4852 (2000).
Rush, J. S., Sweitzer, T., Kent, C., Decker, G. L. & Waechter, C. J. Biogenesis of the endoplasmic reticulum in activated B lymphocytes: temporal relationships between the induction of protein N-glycosylation activity and the biosynthesis of membrane protein and phospholipid. Arch. Biochem. Biophys. 284, 63–70 (1991).
Maccioni, H. J., Daniotti, J. L. & Martina, J. A. Organization of ganglioside synthesis in the Golgi apparatus. Biochim. Biophys. Acta 1437, 101–118 (1999).
Melian, A. et al. Molecular recognition of human CD1b antigen complexes: evidence for a common pattern of interaction with αβ TCRs. J. Immunol. 165, 4494–4504 (2000).
Acknowledgements
We thank M. Sugita, M. Brenner, I. Wilson, T. Cheng, A. Rudensky, K. Honey, V. Cerundolo, Y. Jones and S. Gadola for providing data to illustrate figures and for communicating unpublished data.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
Glossary
- ENDOGLYCOSIDASE-H RESISTANCE
-
Endoglycosidase H is an enzyme that selectively cleaves high-mannose asparagine-linked oligosacccharides. As most glycoproteins are processed from high-mannose to complex oligosaccharides in the Golgi apparatus, the resistance of the glycans of a glycoprotein to cleavage by endoglycosidase H shows that the glycoprotein has entered or passed through the Golgi apparatus.
- CLATHRIN-COATED PIT
-
A membrane invagination that contains transmembrane proteins and a layer of electron-dense clathrin, clathrin adaptor and other proteins on its cytoplasmic face. This structure buds from the membrane to become a clathrin-coated transport vesicle.
- SURFACE PLASMON RESONANCE
-
The detection of alterations in plasmon waves generated at a metal–liquid interface. Changes in surface plasmon resonance are a function of the mass of molecules bound to the interface, so this technique allows sensitive detection of ligand binding in real time without requiring the chemical modification of ligands to enable their detection.
- YEAST TWO-HYBRID
-
A screening system for protein–protein interactions, which result in the transcription of a reporter gene when a bait protein attached to a DNA-binding domain comes into contact with a prey protein bound to a transcriptional activator.
- MANNOSYL PHOSPHOISOPRENOIDS
-
(MPIs). Mycobacterial phospholipids that are characterized by a mannose residue in a β-1 linkage to a terminally phosphorylated polymethylated alkane chain.
- INVARIANT NKT CELLS
-
Lymphocytes that express Vα14 (mice) or Vα24 (human) precisely rearranged to particular Jα gene segments to give T-cell receptor α-chains with an invariant sequence. Typically, they co-express cell-surface markers that are encoded in the natural killer (NK) locus and they are activated by recognition of CD1D, particularly when an α-galactosyl ceramide is bound in its groove.
- α-GALACTOSYL CERAMIDE
-
A synthetic or marine-sponge-derived glycolipid containing an α-glycosidic linkage of the galactose residue to the sphingosine base. This and structurally related lipids potently activate invariant (T-cell receptor Vα14+) natural killer T cells.
- MYCOLIC ACIDS
-
Long-chain fatty acids produced by mycobacteria and related species that are characterized by an alkane branch at the α-carbon and a β-hydroxyl group. Natural mycolyl glycolipids include glucose monomycolate, trehalose mycolate and trehalose dimycolate (cord factor).
- PATTERN-RECOGNITION RECEPTORS
-
Receptors that bind to molecular patterns found in pathogens, but not mammalian cells. Examples include the mannose receptor, which binds terminally mannosylated and polymannosylated compounds, and Toll-like receptors, which are activated by various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA.
Rights and permissions
About this article
Cite this article
Moody, D., Porcelli, S. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol 3, 11–22 (2003). https://doi.org/10.1038/nri979
Issue Date:
DOI: https://doi.org/10.1038/nri979
This article is cited by
-
Phosphatidylinositolmannoside vaccination induces lipid-specific Th1-responses and partially protects guinea pigs from Mycobacterium tuberculosis challenge
Scientific Reports (2023)
-
Identification of a broad lipid repertoire associated to the endothelial cell protein C receptor (EPCR)
Scientific Reports (2022)
-
Mrp1 is involved in lipid presentation and iNKT cell activation by Streptococcus pneumoniae
Nature Communications (2018)
-
Molecular recognition of microbial lipid-based antigens by T cells
Cellular and Molecular Life Sciences (2018)
-
Inhibition of endocytic lipid antigen presentation by common lipophilic environmental pollutants
Scientific Reports (2017)