Key Points
-
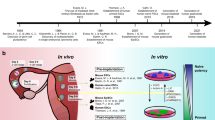
Stem cells have two main properties — self-renewal and multipotentiality.
-
It was shown recently that two independent generation events of haematopoietic stem cells (HSCs) occur in the extra-embryonic (yolk sac) and intra-embryonic compartments.
-
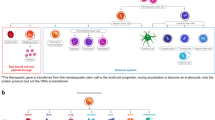
These two types of precursor coexist in early vertebrate embryos. The precursors that originate in the yolk sac are not multipotent and have a limited self-renewal capacity. The other type of precursor, which originates in the intra-embryonic para-aortic region, has both features of HSCs. These observations indicate that self-renewal and multipotentiality cannot be dissociated.
-
Extra-embryonic precursors give rise to specific subsets of mature erythromyeloid cells that have an accelerated differentiation rate compared with the intra-embryonic-derived progenitors. So, haematopoietic development relies on a dual contribution — first, from a fast then exhausted extra-embryonic precursor (the hare), and then from a slowly developing and long-lasting intra-embryonic HSC (the tortoise).
Abstract
Cellular and gene therapy are obvious approaches to correct rare genetic disorders and, in the future, degenerative conditions. Both methods rely on the ability to identify and genetically modify stem cells in vitro, and on the reproducibility of in vivo colonization by the manipulated cells. The basic processes that are involved in the generation of haematopoietic cells in the embryo have been described in the past ten years, as a result of interdisciplinary approaches. These efforts have led to the identification of two independently generated types of haematopoietic progenitor cell, which differ in their potential for self-renewal and differentiation. These two populations might become key tools to understand the properties of stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sabin, F. R. Studies on the origin of blood vessels and of red-blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Carnegie Contrib. Embryol. 272, 214–262 (1920).
Shivdasani, R. A. & Orkin, S. H. The transcriptional control of hematopoiesis. Blood 87, 4025–4039 (1996).
Shivdasani, R. A., Mayer, E. L. & Orkin, S. H. Absence of blood formation in mice lacking the T-cell leukemia oncoprotein TAL-1/SCL. Nature 373, 432–434 (1995).
Robb, L. et al. Absence of yolk-sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl Acad. Sci. USA 92, 7075–7079 (1995).
Robb, L. et al. The Scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15, 4123–4129 (1996).
Porcher, C. et al. The T-cell leukemia oncoprotein SCL/TAL-1 is essential for development of all hematopoietic lineages. Cell 86, 47–57 (1996).
Warren, A. J. et al. The oncogenic cysteine-rich LIM domain protein Rbtn-2 is essential for erythroid development. Cell 78, 45–57 (1994).
Yamada, Y. et al. The T-cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc. Natl Acad. Sci. USA 95, 3890–3895 (1998).
Tsai, F. Y. et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–226 (1994).
Pevny, L. et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349, 257–260 (1991).
Pevny, L. et al. Development of hematopoietic cells lacking transcription factor GATA-1. Development 121, 163–172 (1995).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995).
Shalaby, F. et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 89, 981–990 (1997).
Schuh, A. C., Faloon, P., Hu, Q. L., Bhimani, M. & Choi, K. In vitro hematopoietic and endothelial potential of flk-1−/− embryonic stem cells and embryos. Proc. Natl Acad. Sci. USA 96, 2159–2164 (1999).
Takakura, N. et al. Critical role of the TIE2 endothelial-cell receptor in the development of definitive hematopoiesis. Immunity 9, 677–686 (1998).
Bernstein, A., Forrester, L., Reith, A. D., Dubreuil, P. & Rottapel, R. The murine W/c-kit and Steel loci and the control of hematopoiesis. Semin. Hematol. 28, 138–142 (1991).
Ogawa, M. et al. Expression and function of c-Kit in fetal hemopoietic progenitor cells: transition from the early c-Kit-independent to the late c-Kit-dependent wave of hemopoiesis in the murine embryo. Development 117, 1089–1098 (1993).
Bernex, F. et al. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development 122, 3023–3033 (1996).
Wu, H., Liu, X., Jaenisch, R. & Lodish, H. F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 83, 59–67 (1995).
Lin, C. S., Lim, S. K., D'Agati, V. & Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 10, 154–164 (1996).
Neubauer, H. et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93, 397–409 (1998).
Esposito, G. et al. Disruption of the Rev3l-encoded catalytic subunit of polymerase-ζ in mice results in early embryonic lethality. Curr. Biol. 10, 1221–1224 (2000).
Wang, Q. et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl Acad. Sci. USA 93, 3444–3449 (1996).
Castilla, L. H. et al. Failure of embryonic hematopoiesis and lethal hemorrhages in mouse embryos heterozygous for a knocked-in leukemia gene CBFB-MYH11. Cell 87, 687–696 (1996).
Yergeau, D. A. et al. Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1–ETO fusion gene. Nature Genet. 15, 303–306 (1997).
North, T. et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999).
Mucenski, M. L. et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65, 677–689 (1991).
Wang, Q. et al. The CBFβ subunit is essential for CBFα2 (AML1) function in vivo. Cell 87, 697–708 (1996).
Georgopoulos, K. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 (1994).
Nichogiannopoulou, A., Trevisan, M., Neben, S., Friedrich, C. & Georgopoulos, K. Defects in hemopoietic stem-cell activity in Ikaros-mutant mice. J. Exp. Med. 190, 1201–1214 (1999).
Porter, F. et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain and definitive erythrocyte development. Development 124, 2935–2944 (1997).
Grossmann, M. et al. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl Acad. Sci. USA 96, 11848–11853 (1999).
Kitajima, K. et al. Definitive but not primitive hematopoiesis is impaired in jumonji mutant mice. Blood 93, 87–95 (1999).
Scott, E. W. et al. PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid–myeloid progenitors. Immunity 6, 437–447 (1997).
Hirsch, E., Iglesias, A., Potocnik, A. J., Hartmann, U. & Fassler, R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature 380, 171–175 (1996).
Potocnik, A. J., Brakebusch, C. & Fassler, R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen and bone marrow. Immunity 12, 653–663 (2000).
Yang, J. T., Rayburn, H. & Hynes, R. O. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development 121, 549–560 (1995).
Arroyo, A. G., Yang, J. T., Rayburn, H. & Hynes, R. O. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell 85, 997–1008 (1996).
Arroyo, A. G., Yang, J. T., Rayburn, H. & Hynes, R. O. α4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity 11, 555–566 (1999).
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC-chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Nagasawa, T., Tachibana, K. & Kishimoto, T. A novel CXC-chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin. Immunol. 10, 179–185 (1998).
Silver, L. & Palis, J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 89, 1154–1164 (1997).
Kennedy, M. et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386, 488–493 (1997).
Lee, R., Kertesz, N., Joseph, S. B., Jegalian, A. & Wu, H. Erythropoietin (Epo) and EpoR expression and two waves of erythropoiesis. Blood 98, 1408–1415 (2001).
Naito, M. et al. Development, differentiation and phenotypic heterogeneity of murine tissue macrophages. J. Leukocyte Biol. 59, 133–138 (1996).
Xu, M. J. et al. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood 97, 2016–2022 (2001).
Metcalf, D. & Moore, M. A. S. in Haematopoietic Cells (eds Neuberger, A. & Tatum, E. L.) 173–271 (North Holland Publishing, Amsterdam, 1971).
Houssaint, E. Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell. Differ. 10, 243–252 (1981).
Fontaine-Perrus, J. C., Calman, F. M., Kaplan, C. & Le Douarin, N. M. Seeding of the 10-day mouse embryo thymic rudiment by lymphocyte precursors in vitro. J. Immunol. 126, 2310–2316 (1981).
Manaia, A. et al. Lmo2 and GATA-3 associated expression in intra-embryonic hemogenic sites. Development 127, 643–653 (2000).
Godin, I., Garcia-Porrero, J. A., Dieterlen-Lievre, F. & Cumano, A. Stem-cell emergence and hemopoietic activity are incompatible in mouse intra-embryonic sites. J. Exp. Med. 190, 43–52 (1999).
Sabin, F. R. Origin and development of the primitive vessels of the chick and of the pig. Carnegie Contrib. Embryol. 18, 61–124 (1917).
Murray, P. The development in vitro of blood of the early chick embryo. Proc. R. Soc. 111, 497–521 (1932).
Robb, L. & Elefanty, A. G. The hemangioblast — an elusive cell captured in culture. Bioessays 20, 611–614 (1998).
Robertson, S., Kennedy, M. & Keller, G. Hematopoietic commitment during embryogenesis. Ann. NY Acad. Sci. 872, 9–15 (1999).
Weissman, I., Papaioannou, V. & Gardner, R. in Differentiation of Normal and Neoplastic Cells (eds Clarkson, B., Mark, P. & Till, J.) 33–47 (Cold Spring Harbour Laboratory Press, New York, 1978).
Toles, J. F., Chui, D. H., Belbeck, L. W., Starr, E. & Barker, J. E. Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc. Natl Acad. Sci. USA 86, 7456–7459 (1989).
Le Douarin, N. M. Interspecific cell markers and cell lineage in birds. Phil. Trans. R. Soc. Lond. B 312, 153–162 (1985).
Dieterlen-Lièvre, F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J. Embryol. Exp. Morphol. 33, 607–619 (1975).
Dieterlen-Lièvre, F. & Martin, C. Diffuse intra-embryonic hemopoiesis in normal and chimeric avian development. Dev. Biol. 88, 180–191 (1981).
Cormier, F. & Dieterlen-Lièvre, F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development 102, 279–285 (1988).
Turpen, J. B. Induction and early development of the hematopoietic and immune systems in Xenopus. Dev. Comp. Immunol. 22, 265–278 (1998).
Bechtold, T. E., Smith, P. B. & Turpen, J. B. Differential stem-cell contributions to thymocyte succession during development of Xenopus laevis. J. Immunol. 148, 2975–2982 (1992).
Ciau-Uitz, A., Walmsley, M. & Patient, R. Distinct origins of adult and embryonic blood in Xenopus. Cell 102, 787–796 (2000).The independent origin of extra-embryonic and intra-embryonic haematopoietic precursors is timed back to the early stages of development in amphibian embryos.
Tavian, M., Hallais, M. F. & Peault, B. Emergence of intra-embryonic hematopoietic precursors in the pre-liver human embryo. Development 126, 793–803 (1999).
Cumano, A., Dieterlen-Lièvre, F. & Godin, I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intra-embryonic splanchnopleura. Cell 86, 907–916 (1996).A direct demonstration of the independent origin of extra-embryonic and intra-embryonic haematopoietic precursors in mouse embryos. Evidence for distinct differentiation potentials of precursors isolated from the two sites.
Cumano, A., Ferraz, J. C., Klaine, M., Di Santo, J. P. & Godin, I. Intra-embryonic, but not yolk-sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15, 477–485 (2001).Elucidation of a mechanism that is involved in the rejection of embryonic cells after adult engraftment.
Tavian, M., Robin, C., Coulombel, L. & Peault, B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intra-embryonic mesoderm. Immunity 15, 487–495 (2001).Demonstration of the conservation of haematopoietic-development processes between mouse and human embryos.
Godin, I., Dieterlen-Lièvre, F. & Cumano, A. Emergence of multipotent hematopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryo, beginning at 8.5 days postcoitus. Proc. Natl Acad. Sci. USA 92, 773–777 (1995).
Nishikawa, S. I. et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8, 761–769 (1998).An analysis of lineage relationships leading to the intra-embryonic emergence of haematopoietic stem cells.
Ozato, K., Wan, Y. J. & Orrison, B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc. Natl Acad. Sci. USA 82, 2427–2431 (1985).
Jaffe, L., Robertson, E. J. & Bikoff, E. K. Distinct patterns of expression of MHC class I and β2-microglobulin transcripts at early stages of mouse development. J. Immunol. 147, 2740–2749 (1991).
Colucci, F. et al. Dissecting NK-cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK-cell differentiation. J. Immunol. 162, 2761–2765 (1999).
Muller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F. & Dzierzak, E. Development of hematopoietic stem-cell activity in the mouse embryo. Immunity 1, 291–301 (1994).First demonstration that the aorta–gonad–mesonephros (AGM) region harbours haematopoietic stem cells.
Medvinsky, A. & Dzierzak, E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906 (1996).The AGM region is able to produce and/or expand haematopoietic stem-cell populations.
Delassus, S. & Cumano, A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity 4, 97–106 (1996).
Yoder, M. C. & Hiatt, K. Engraftment of embryonic hematopoietic cells in conditioned newborn recipient. Blood 89, 2176–2183 (1997).
Spangrude, G. J., Heimfield, D. S. & Weissman, I. L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
de Bruijn, M. et al. Hematopoietic stem cells localize to the endothelial layer in the midgestation mouse aorta. Immunity 16, 673–683 (2002).
Sanchez, M. J., Holmes, A., Miles, C. & Dzierzak, E. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity 5, 513–525 (1996).
Jordan, C. T. et al. Long-term repopulating abilities of enriched fetal liver stem cells measured by competitive repopulation. Exp. Hematol. 23, 1011–1015 (1995).
de Bruijn, M. F., Speck, N. A., Peeters, M. C. & Dzierzak, E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474 (2000).
Garcia-Porrero, J. A., Godin, I. E. & Dieterlen-Lièvre, F. Potential intra-embryonic hemogenic sites at preliver stages in the mouse. Anat. Embryol. 192, 425–435 (1995).
Tavian, M. et al. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood 87, 67–72 (1996).
Pardanaud, L., Yassine, F. & Dieterlen-Lièvre, F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development 105, 473–485 (1989).
Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575–4583 (1998).
Thompson, M. A. et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 197, 248–269 (1998).
Marshall, C. J. et al. Detailed characterization of the human aorta–gonad–mesonephros region reveals morphological polarity resembling a hematopoietic stromal layer. Dev. Dyn. 215, 139–147 (1999).
Marshall, C. J., Kinnon, C. & Thrasher, A. J. Polarized expression of bone morphogenetic protein-4 in the human aorta–gonad–mesonephros region. Blood 96, 1591–1593 (2000).
Matsuoka, S. et al. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta–gonad–mesonephros region-derived stromal cells. Blood 98, 6–12 (2001).
Emmel, V. The cell clusters in the dorsal aorta of mammalian embryos. Am. J. Anat. 401–421 (1916).
Smith, R. A. & Glomski, C. A. 'Hemogenic endothelium' of the embryonic aorta: does it exist? Dev. Comp. Immunol. 6, 359–368 (1982).
Nishikawa, S. I. et al. All B cells are progeny of endothelial cells: a new perspective. Immunol. Rev. 175, 112–119 (2000).
Hamaguchi, I. et al. In vitro hematopoietic and endothelial-cell development from cells expressing TEK receptor in murine aorta–gonad–mesonephros region. Blood 93, 1549–1556 (1999).
Drake, C. J. & Fleming, P. A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 (2000).
Dumont, D. J. et al. Vascularization of the mouse embryo: a study of Flk-1, Tek, Tie and vascular endothelial growth factor expression during development. Dev. Dyn. 203, 80–92 (1995).
Jaffredo, T., Gautier, R., Brajeul, V. & Dieterlen-Lievre, F. Tracing the progeny of the aortic hemangioblast in the avian embryo. Dev. Biol. 224, 204–214 (2000).
Hara, T. et al. Identification of podocalyxin-like protein 1 as a novel cell-surface marker for hemangioblasts in the murine aorta–gonad–mesonephros region. Immunity 11, 567–578 (1999).
Nishikawa, S. I., Nishikawa, S., Hirashima, M., Matsuyoshi, N. & Kodama, H. Progressive lineage analysis by cell sorting and culture identifies FLK1+cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747–1757 (1998).
Ogawa, M. et al. Expression of α4-integrin defines the earliest precursor of hematopoietic-cell lineage diverged from endothelial cells. Blood 93, 1168–1177 (1999).
Robertson, S. M., Kennedy, M., Shannon, J. M. & Keller, G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/TAL-1. Development 127, 2447–2459 (2000).
Yamashita, J. et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000).An analysis of differentiation steps leading from embryonic-stem-cell-derived mesoderm to various lineages. An in vivo assay allows extended evaluation of the differentiation potential of mesodermal cells.
Cai, Z. et al. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 13, 423–431 (2000).
Kyba, M., Perlingeiro, R. C. & Daley, G. Q. HoxB4 confers definitive lymphoid–myeloid engraftment potential on embryonic stem cell and yolk-sac hematopoietic progenitors. Cell 109, 29–37 (2002).
Antonchuk, J., Sauvageau, G. & Humphries, R. K. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109, 39–45 (2002).
Rideout, W. M., Hochedlinger, K., Kyba, M., Daley, G. Q. & Jaenisch, R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell 109, 17–27 (2002).The first direct evidence that in vitro -differentiated ES cells can reconstitute irradiated recipients, and the first comprehensive attempt at corrective cell therapy.
Pevny, L. et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349, 257–260 (1991).
Oike, Y. et al. Mice homozygous for a truncated form of CREB-binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood 93, 2771–2779 (1999).
Jepsen, K. et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102, 753–763 (2000).
Marine, J. C. et al. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell 98, 617–627 (1999).
Ody, C., Vaigot, P., Quere, P., Imhof, B. A. & Corbel, C. Glycoprotein IIb–IIIa is expressed on avian multilineage hematopoietic progenitor cells. Blood 93, 2898–2906 (1999).
Watt, S. M. et al. Functionally defined CD164 epitopes are expressed on CD34+ cells throughout ontogeny but display distinct distribution patterns in adult hematopoietic and nonhematopoietic tissues. Blood 95, 3113–3124 (2000).
Labastie, M. C., Cortes, F., Romeo, P. H., Dulac, C. & Peault, B. Molecular identity of hematopoietic precursor cells emerging in the human embryo. Blood 92, 3624–3635 (1998).
Vandenbunder, B., Pardanaud, L., Jaffredo, T., Mirabel, M. A. & Stehelin, D. Complementary patterns of expression of c-ets1, c-myb and c-myc in the blood-forming system of the chick embryo. Development 107, 265–274 (1989).
Cortès, F., Debacker, C., Peault, B. & Labastie, M. C. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech. Dev. 83, 161–164 (1999).
Garcia-Porrero, J. A. et al. Antigenic profiles of endothelial and hemopoietic lineages in murine intra-embryonic hemogenic sites. Dev. Comp. Immunol. 22, 303–319 (1998).
Wood, H. B., May, G., Healy, L., Enver, T. & Morriss Kay, G. M. CD34 expression patterns during early mouse development are related to modes of blood-vessel formation and reveal additional sites of hematopoiesis. Blood 90, 2300–2311 (1997).
Gering, M., Rodaway, A. R., Gottgens, B., Patient, R. K. & Green, A. R. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17, 4029–4045 (1998).
Brachtendorf, G. et al. Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev. Dyn. 222, 410–419 (2001).
Yoshida, H. et al. Hematopoietic tissues, as a playground of receptor tyrosine kinases of the PDGF-receptor family. Dev. Comp. Immunol. 22, 321–332 (1998).
North, T. E. et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661–672 (2002).
Petrenko, O. et al. The molecular characterization of the fetal stem-cell marker AA4. Immunity 10, 691–700 (1999).
Anstrom, K. K. & Tucker, R. P. Tenascin-C lines the migratory pathways of avian primordial germ cells and hematopoietic progenitor cells. Dev. Dyn. 206, 437–446 (1996).
Cortes, F. et al. HCA, an immunoglobulin-like adhesion molecule present on the earliest human hematopoietic precursor cells, is also expressed by stromal cells in blood-forming tissues. Blood 93, 826–837 (1999).
Debacker, C., Catala, M. & Labastie, M. C. Embryonic expression of the human GATA-3 gene. Mech. Dev. 85, 183–187 (1999).
Neave, B., Rodaway, A., Wilson, S. W., Patient, R. & Holder, N. Expression of zebrafish GATA3 (gta3) during gastrulation and neurulation suggests a role in the specification of cell fate. Mech. Dev. 51, 169–182 (1995).
Acknowledgements
I.G. and A.C. are supported by grants from the French Ministry of Research (ACI developmental biology), and I.G. is supported by a grant from the 'Association pour la Recherche sur le Cancer'. The 'Unité du Développement des Lyphocytes' is supported by the 'Ligue Nationale de la Recherche contre le Cancer' as a registered laboratory.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
Glossary
- HAEMATOPOIESIS
-
The commitment and differentiation processes that lead from a haematopoietic stem cell to the production of mature cells of all lineages — erythrocytes, myeloid cells (macrophages, mast cells, neutrophils and eosinophils), B and T cells, and natural killer cells.
- HAEMATOPOIETIC STEM CELLS
-
Cells that have the ability to both generate all types of haematopoietic cell (multipotentiality) and replace themselves (self-renewal) throughout the lifetime of an individual. Whereas multipotentiality can be assessed in vitro and in vivo, self-renewal can be determined only by the in vivo detection of long-term reconstitution activity.
- PRIMITIVE/DEFINITIVE
-
This term refers to specific haematopoietic lineages that arise early or late during embryogenesis or to early/late phases of the haematopoietic process.
- SPECIFIC LINEAGES
-
Mature cells are qualified as 'primitive' when they have features that are present only in early embryos — for example, primitive erythrocytes synthesize a type of globin that is expressed only during ontogeny. Mature cells that have the same characteristics irrespective of whether they are present in embryos or adults are known as 'definitive'.
- WHOLE PROCESS OF HAEMATOPOIESIS
-
Primitive haematopoiesis refers to blood-cell production originating from the yolk sac. Definitive haematopoiesis is carried out in the fetal liver in the embryo, from haematopoietic stem cells that are generated initially in the splanchnopleura/aorta–gonad–mesonephros, and then in the bone marrow of adults.
- ERYTHROPOIESIS
-
The differentiation process leading from a committed erythroid precursor to the production of mature red blood cells (erythrocytes).
- AORTA–GONAD–MESONEPHROS
-
(AGM). The embryonic site at which definitive haematopoietic stem cells (HSCs) are produced. It comprises the aorta, and developing reproductive and excretory (mesonephros) systems. Within this haemogenic site, HSCs are concentrated in the aorta region.
- MONOCYTE
-
An intermediate cell type in the pathway leading from a myeloid precursor to a differentiated macrophage.
- PLOIDY
-
The DNA content of the nucleus, which is usually 2N. Megakaryocytes synthesize DNA without cytokinesis. In the adult, their ploidy is 16N.
- HAEMOGENIC SITE
-
The site at which de novo generation of haematopoietic cells occurs. Two haemogenic sites have been identified in the embryo — the yolk sac and an intra-embryonic site, the splanchnopleura/aorta–gonad–mesonephros region.
- SOMITE
-
Somites are the result of bilateral cell condensation in the dorsal body wall of vertebrate embryos. The somitic cells are founders of the skeletal muscle of the body wall, the back and the limbs, of the dermis, and of the vertebrae and ribs. Somite counting is a precise way to assess the developmental stage of a rapidly changing embryo, because an additional pair of somites are added about every two hours.
- HAEMANGIOBLAST
-
A presumptive cell type that can give rise to endothelial cells and haematopoietic cells. The existence of these bi-lineage precursors, still not proved formally, was inferred from the observation that in the early-embryo yolk sac, blood vessels and circulating cells arise from apparently homogeneous cell clusters.
- BLASTOMERE
-
A cell that results from the cleavage of the zygote.
- LONG-TERM RECONSTITUTION (LTR) ACTIVITY
-
The capacity of a haematopoietic precursor to reconstitute all lineages for more than six months (long term) when injected into an irradiated mouse. It reveals the self-renewal capacity of haematopoietic stem cells.
Rights and permissions
About this article
Cite this article
Godin, I., Cumano, A. The hare and the tortoise: an embryonic haematopoietic race. Nat Rev Immunol 2, 593–604 (2002). https://doi.org/10.1038/nri857
Issue Date:
DOI: https://doi.org/10.1038/nri857
This article is cited by
-
Phosphatidylinositol-3 kinase signaling controls survival and stemness of hematopoietic stem and progenitor cells
Oncogene (2021)
-
Yolk sac macrophage progenitors traffic to the embryo during defined stages of development
Nature Communications (2018)
-
Interplay of transcription factors and microRNAs during embryonic hematopoiesis
Science China Life Sciences (2017)
-
Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators
Nature Communications (2014)
-
Generating parabiotic zebrafish embryos for cell migration and homing studies
Nature Methods (2013)