Key Points
-
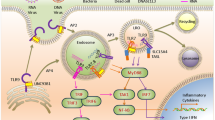
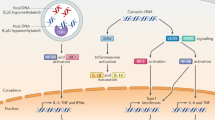
Endosomal and cytosolic nucleic acid receptors sense microbial nucleic acids and initiate innate immune responses. However, in some circumstances their activation by endogenous nucleic acids can also contribute to autoinflammation.
-
Nucleic acid sensors and their signalling pathways constitute promising drug targets for which synthetic oligonucleotide drugs as well as low-molecular-weight compounds are currently being developed.
-
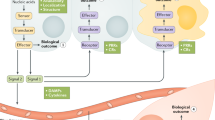
Agonists of nucleic acid sensors function as immunostimulants and have uses in cancer immunotherapy or as vaccine adjuvants. The key challenge is to limit systemic inflammation.
-
Antagonists are being developed as immunomodulators for autoimmune diseases including systemic lupus erythematosus and psoriasis, and for autoinflammatory conditions (for example, type I interferonopathies). The key challenge is to design clinical proof-of-concept studies in which the pharmacological profile of antagonists is matched to the molecular phenotype of patients.
-
In vitro use of these compounds and results from animal studies as well as ongoing clinical trials are leading to a better molecular understanding of these indications. This, in turn, enables further drug discovery efforts for better therapy of diseases with high unmet medical need.
Abstract
Nucleic acid sensing by innate receptors initiates immune defences against viruses and other pathogens. A hallmark of this response is the release of interferons (IFNs), which promote protective immunity by inducing IFN-stimulated genes (ISGs). A similar ISG signature is found in autoinflammatory and autoimmune conditions, indicating that chronic activation of nucleic acid-sensing pathways may contribute to these diseases. Here, we review how nucleic acid-sensing pathways are currently being targeted pharmacologically with both agonists and antagonists. We discuss how an improved understanding of the biology of these pathways is leading to novel therapies for infections, cancer, and autoimmune and autoinflammatory disorders, and how new therapeutics will, in turn, generate a deeper understanding of these complex diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005).
Egli, A., Santer, D. M., O'Shea, D., Tyrrell, D. L. & Houghton, M. The impact of the interferon-λ family on the innate and adaptive immune response to viral infections. Emerg. Microbes Infect. 3, e51 (2014).
Schneider, W. M., Chevillotte, M. D. & Rice, C. M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 32, 513–545 (2014).
Pichlmair, A. & Reis, E. Sousa, C. Innate recognition of viruses. Immunity 27, 370–383 (2007).
García-Sastre, A. 2 methylate or not 2 methylate: viral evasion of the type I interferon response. Nat. Immunol. 12, 114–115 (2011).
Morita, M. et al. Gene-targeted mice lacking the trex1 (DNase III) 3′→5′ DNA exonuclease develop inflammatory myocarditis. Mol. Cell. Biol. 24, 6719–6727 (2004).
Yoshida, H., Okabe, Y., Kawane, K., Fukuyama, H. & Nagata, S. Lethal anemia caused by interferon-β produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6, 49–56 (2005).
Ahn, J., Ruiz, P. & Barber, G. N. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J. Immunol. 193, 4634–4642 (2014).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
Lan, Y. Y., Londono, D., Bouley, R., Rooney, M. S. & Hacohen, N. Dnase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Rep. 9, 180–192 (2014).
Chan, M. P. et al. DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat. Commun. 6, 5853 (2015).
Pawaria, S. et al. Cutting edge: DNase II deficiency prevents activation of autoreactive B cells by double-stranded DNA endogenous ligands. J. Immunol. 194, 1403–1407 (2015).
Woo, S.-R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014). This study was the first to indicate a crucial role for cytosolic sensing of self DNA in tumour surveillance.
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007). This was the first paper to demonstrate that an antimicrobial peptide from psoriatic skin shuttles DNA into cells and induces type I IFN release via TLR9.
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004). This paper demonstrates that sera from patients with SLE can form immune complexes with necrotic material and induce type I IFN release from pDCs.
Bave, U. et al. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 52, 1185–1195 (2005).
Blasius, A. L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Ohto, U. et al. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 520, 702–705 (2015). References 18 and 19 provided the first structural insight into unexpected aspects of single-stranded nucleic acid ligand binding by TLR8 and TLR9, respectively. TLRs have proven notoriously difficult to express and crystallize.
Hornung, V. SnapShot: nucleic acid immune sensors, part 1. Immunity 41, 868–868. e1 (2014).
Hornung, V. et al. 5′-triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Goubau, D. et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514, 372–375 (2014).
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 (2008).
Pichlmair, A. et al. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 83, 10761–10769 (2009).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Wu, J. et al. Cyclic GMP–AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013). References 26 and 27 report the seminal discovery of the cytosolic DNA receptor, as well as the small-molecule second messenger, that links to STING activation.
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Jin, L. et al. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 187, 2595–2601 (2011).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015). This study highlights a previously unappreciated role for adaptor phosphorylation as an integral step in the activation of nucleic acid signalling pathways.
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Huen, A. O. & Rook, A. H. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr. Opin. Oncol. 26, 237–244 (2014).
Kasturi, S. P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Kastenmüller, K. et al. Protective T cell immunity in mice following protein–TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Invest. 121, 1782–1796 (2011).
Ilyinskii, P. O. et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 32, 2882–2895 (2014).
Kastenmuller, W., Kastenmüller, K., Kurts, C. & Seder, R. A. Dendritic cell-targeted vaccines — hope or hype? Nat. Rev. Immunol. 14, 705–711 (2014).
Field, A. K., Tytell, A. A., Lampson, G. P. & Hilleman, M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl Acad. Sci. USA 58, 1004–1010 (1967).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl Acad. Sci. USA 103, 8459–8464 (2006).
Caskey, M. et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 208, 2357–2366 (2011).
Ammi, R. et al. Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol. Ther. 140, 120–131 (2014).
Strayer, D. R. et al. A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS ONE 7, e31334 (2012).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 (2002).
Baguley, B. C. & Ching, L. M. DMXAA: an antivascular agent with multiple host responses. Int. J. Radiat. Oncol. Biol. Phys. 54, 1503–1511 (2002).
Lara, P. N. et al. Randomized Phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2965–2971 (2011).
Conlon, J. et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 190, 5216–5225 (2013).
Gao, P. et al. Binding-pocket and lid-region substitutions render human STING sensitive to the species-specific drug DMXAA. Cell Rep. 8, 1668–1676 (2014).
Karaolis, D. K. R. et al. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 178, 2171–2181 (2007).
Dubensky, T. W., Kanne, D. B. & Leong, M. L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 1, 131–143 (2013).
Gao, P. et al. Structure–function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154, 748–762 (2013).
Li, X.-D. et al. Pivotal roles of cGAS–cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Scheiermann, J. & Klinman, D. M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 32, 6377–6389 (2014).
Bedard, K. M. et al. Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J. Virol. 86, 7334–7344 (2012).
Wang, Y. et al. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat. Struct. Mol. Biol. 17, 781–787 (2010).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Querec, T. et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203, 413–424 (2006).
Coffman, R. L., Sher, A. & Seder, R. A. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010).
Goff, P. H. et al. Synthetic TLR4 and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained and broadly protective responses. J. Virol. 89, 3221–3335 (2015).
Temizoz, B. et al. TLR9 and STING agonists synergistically induce innate and adaptive type II IFN. Eur. J. Immunol. 45, 1159–1169 (2015).
Marshak-Rothstein, A. & Rifkin, I. R. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25, 419–441 (2007). A comprehensive review of the role of endosomal TLRs in SLE,with a particular focus on B cell biology.
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Kuznik, A. et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186, 4794–4804 (2011).
Sun, X. et al. Increased ribonuclease expression reduces inflammation and prolongs survival in TLR7 transgenic mice. J. Immunol. 190, 2536–2543 (2013).
Kalunian, K. et al. Efficacy and safety of rontalizumab (anti-interferon α) in SLE subjects with restricted immunosuppressant use: results of a randomized, double-blind, placebo-controlled Phase 2 study. Arthritis Rheumatol. Abstr. Suppl. 64, S1111 (2012). A conference abstract showing that ISGs can be used for patient stratification in clinical studies.
Blomberg, S. et al. Presence of cutaneous interferon-α producing cells in patients with systemic lupus erythematosus. Lupus 10, 484–490 (2001).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon- α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Berghofer, B. et al. TLR7 ligands induce higher IFN-α production in females. J. Immunol. 177, 2088–2096 (2006).
Laffont, S. et al. X-chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J. Immunol. 193, 5444–5452 (2014).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl Med. 3, 73ra19 (2011).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Lau, C. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005).
Chan, R. W. et al. Plasma DNA aberrations in systemic lupus erythematosus revealed by genomic and methylomic sequencing. Proc. Natl Acad. Sci. USA 111, E5302–E5311 (2014).
Vollmer, J. et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 (2005).
Annable, T. et al. Using poly I:C as an adjuvant does not induce or exacerbate models of systemic lupus erythematosus. Autoimmunity 48, 29–39 (2015).
Deane, J. A. et al. Control of TLR7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Savarese, E. et al. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 58, 1107–1115 (2008).
Demaria, O. et al. TLR8 deficiency leads to autoimmunity in mice. J. Clin. Invest. 120, 3651–3662 (2010).
Wang, J. et al. The functional effects of physical interactions among Toll-like receptors 7, 8, and 9. J. Biol. Chem. 281, 37427–37434 (2006).
Fukui, R. et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity 35, 69–81 (2011).
Nickerson, K. M. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848 (2010).
Desnues, B. et al. TLR8 on dendritic cells and TLR9 on B cells restrain TLR7-mediated spontaneous autoimmunity in C57BL/6 mice. Proc. Natl Acad. Sci. USA 111, 1497–1502 (2014).
Wu, H. J. et al. Inflammatory arthritis can be reined in by CpG-induced DC–NK cell cross talk. J. Exp. Med. 204, 1911–1922 (2007).
Fallarino, F. et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J. Immunol. 183, 6303–6312 (2009).
Wingender, G. et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur. J. Immunol. 36, 12–20 (2006).
Moseman, E. A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173, 4433–4442 (2004).
Miles, K. et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc. Natl Acad. Sci. USA 109, 887–892 (2012).
Pawar, R. D. et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 18, 1721–1731 (2007).
Barrat, F. J., Meeker, T., Chan, J. H., Guiducci, C. & Coffman, R. L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 37, 3582–3586 (2007).
Zhu, F. G. et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity 46, 419–428 (2013).
Guiducci, C. et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465, 937–941 (2010).
van der Fits, L., van der Wel, L. I., Laman, J. D., Prens, E. P. & Verschuren, M. C. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-α sensitivity is unaltered. J. Investig. Dermatol. 122, 51–60 (2004).
Ganguly, D. et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994 (2009).
Yu, D. et al. Modifications incorporated in CpG motifs of oligodeoxynucleotides lead to antagonist activity of Toll-like receptors 7 and 9. J. Med. Chem. 52, 5108–5114 (2009).
Suarez-Farinas, M. et al. Treatment of psoriasis patients with IMO-3100 shows improvement in gene expression patterns of meta-analysis derived-3 transcriptome and IL-17 pathway. Arthritis Rheumatol. Abstr. Suppl. 65, S495 (2013).
Langley, R. G. et al. Secukinumab in plaque psoriasis — results of two Phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Suarez-Farinas, M. et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS ONE 8, e84634 (2013).
Acosta-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Balak, D. M. W. et al. Results from a randomized, double-blind, placebo-controlled, monotherapy trial of IMO-8400 demonstrate clinical proof-of-concept for Toll-like receptor 7. 8 and 9 antagonism in psoriasis (poster). 73rd Annual Meeting of the American Academy of Dermatology 1805 (2015).
Ngo, V. N. et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011).
Wang, J. Q., Jeelall, Y. S., Beutler, B., Horikawa, K. & Goodnow, C. C. Consequences of the recurrent MYD88L265P somatic mutation for B cell tolerance. J. Exp. Med. 211, 413–426 (2014).
Crow, Y. J. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr. Opin. Immunol. 32, 7–12 (2015).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′–5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Rice, G. I. et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 (2014).
Funabiki, M. et al. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40, 199–212 (2014).
Crampton, S. P., Deane, J. A., Feigenbaum, L. & Bolland, S. Ifih1 gene dose effect reveals MDA5- mediated chronic type I IFN gene signature, viral resistance, and accelerated autoimmunity. J. Immunol. 188, 1451–1459 (2012).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Sharma, S. et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl Acad, Sci. USA 112, E710–E717 (2015). A provocative study showing that STING deficiency in mice unexpectedly exacerbates lupus-like disease.
Huang, L. et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 191, 3509–3513 (2013).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013).
Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1361 (2013).
Kranzusch, P. J., Lee, A. S.-Y., Berger, J. M. & Doudna, J. A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 3, 1362–1368 (2013).
Lamphier, M. et al. Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo. Mol. Pharmacol. 85, 429–440 (2014).
Baum, R. et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J. Immunol. 194, 873–877 (2015).
Gehrke, N. et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495 (2013).
Yu, P. et al. Nucleic acid-sensing toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity 37, 867–879 (2012).
Ciancanelli, M. J. et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348, 448–453 (2015).
Alsina, L. et al. A narrow repertoire of transcriptional modules responsive to pyogenic bacteria is impaired in patients carrying loss-of-function mutations in MYD88 or IRAK4. Nat. Immunol. 15, 1134–1142 (2014).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Zeng, M. et al. MAVS, cGAS, and endogenous retroviruses in T-independent B cell responses. Science 346, 1486–1492 (2014).
Kuemmerle-Deschner, J. B. et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res. Ther. 13, R34 (2011).
Ruperto, N. et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 367, 2396–2406 (2012).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015). References 9, 123 and 127 uncover physiological circumstances in which mitochondrial DNA is released as a cell-intrinsic danger signal leading to cGAS-mediated immune activation.
Khamashta, M. et al. Safety and efficacy of sifalimumab, an anti IFN-α monoclonal antibody, in a Phase 2b study of moderate to severe systemic lupus erythematosus (SLE). Arthritis Rheum. 66, 3531 (2014).
Morehouse, C. et al. Target modulation of a type I interferon (IFN) gene signature with sifalimumab or anifrolumab in systemic lupus erythematosis (SLE) patients in two open label Phase 2 Japanese trials. Arthritis Rheumatol. Abstr. Suppl. 66, S313–S314 (2014).
Lauwerys, B. R. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 65, 447–456 (2013).
Zimmer, R., Scherbarth, H. R., Rillo, O. L., Gomez-Reino, J. J. & Muller, S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled Phase IIb clinical trial. Ann. Rheum. Dis. 72, 1830–1835 (2013).
Reilly, S. M. et al. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat. Med. 19, 313–321 (2013).
Chaudhary, D., Robinson, S. & Romero, D. L. Recent advances in the discovery of small molecule inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4) as a therapeutic target for inflammation and oncology disorders. J. Med. Chem. 58, 96–110 (2015).
Balasubramanian, W. R. et al. Novel IRAK-4 inhibitors exhibit highly potent anti-proliferative activity in DLBCL cell lines with activating MYD88 L265P mutation. AACR Annual Meeting Abstract 3646 (2015).
Vajda, E. G., Niecestro, R., Zhi, L. & Marschke, K. B. IRAK4 inhibitors display synergistic activity when combined with BTK or PI3K inhibitors in B cell lymphomas. AACR Annual Meeting Abstract 785 (2015).
Laska, M. J. et al. Polymorphisms within Toll-like receptors are associated with systemic lupus erythematosus in a cohort of Danish females. Rheumatol. 53, 48–55 (2014).
Assmann, T. S., Brondani Lde, A., Bauer, A. C., Canani, L. H. & Crispim, D. Polymorphisms in the TLR3 gene are associated with risk for type 1 diabetes mellitus. Eur. J. Endocrinol. 170, 519–527 (2014).
Garcia-Ortiz, H. et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann. Rheum. Dis. 69, 1861–1865 (2010).
Kawasaki, A. et al. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res. Ther. 13, R41 (2011).
Piotrowski, P., Lianeri, M., Wudarski, M., Olesinska, M. & Jagodzinski, P. P. Contribution of toll-like receptor 9 gene single-nucleotide polymorphism to systemic lupus erythematosus. Rheumatol. Int. 33, 1121–1125 (2013).
Cen, H. et al. Association of IFIH1 rs1990760 polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Autoimmunity 46, 455–462 (2013).
Tansley, S. L. et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res. Ther. 16, R138 (2014).
Nejentsev, S., Walker, N., Riches, D., Egholm, M. & Todd, J. A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 (2009).
Shigemoto, T. et al. Identification of loss of function mutations in human genes encoding RIG-I and mda5: implications for resistance to type I diabetes. J. Biol. Chem. 284, 13348–13354 (2009).
Crow, Y. J. et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. A167A, 296–312 (2015). A comprehensive review on AGS and its underlying genetic causes.
Ravenscroft, J. C., Suri, M., Rice, G. I., Szynkiewicz, M. & Crow, Y. J. Autosomal dominant inheritance of a heterozygous mutation in SAMHD1 causing familial chilblain lupus. Am. J. Med. Genet. A 155A, 235–237 (2011).
Richards, A. et al. C-terminal truncations in human 3′–5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat. Genet. 39, 1068–1070 (2007).
Rutsch, F. et al. A specific IFIH1 gain-of-function mutation causes Singleton–Merten syndrome. Am. J. Hum. Genet. 96, 275–282 (2015).
Jang, M. A. et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton–Merten syndrome. Am. J. Hum. Genet. 96, 266–274 (2015).
Zhang, X. et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 517, 89–93 (2015).
Eckard, S. C. et al. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 15, 839–845 (2014).
Belot, A. et al. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr. Rheumatol. Online J. 12, 44 (2014).
Briggs, T. A. et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat. Genet. 43, 127–131 (2011).
Schuberth-Wagner, C. et al. A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1-2′O-methylated self RNA. Immunity 43, 41–51 (2015).
Mannion N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Reports 9, 1482–1494 (2015).
Liddicoat B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science http://dx.doi.org/10.1126/science.aac7049 (2015).
Acknowledgements
The authors thank E. Bartok, J. Deane, M. Hasan, P. Lötscher, D. Patel, A. Marshak-Rothstein and A. Weber for discussion and helpful suggestions relating to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T.J. is an employee of Novartis Pharma, AG. W.B. declares no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Pathophenotype
-
A disease subtype within a complex disease that is distinguished by certain clinical symptoms. Complex diseases such as systemic lupus erythematosus contain multiple pathophenotypes. The key challenge of molecular pathology is to match pathophenotypes to the activation of specific pathogenic pathways.
- Antinuclear antibodies
-
(ANAs). Autoantibodies against double-stranded DNA or RNA-containing antigens (for example, Sjögren syndrome-related antigen A (SS-A; also known as Ro), SS-B (also known as La), Sm (spliceosomal) and small nuclear ribonucleoprotein 70 kDa (snRNP70)). Their presence in patient sera is a diagnostic hallmark of autoimmune diseases such as Sjögren syndrome and systemic lupus erythematosus. One diagnostic test for ANAs relies on specific staining pattern of cell nuclei with patient sera by immunofluorescence, hence the name.
- Biomarkers
-
Measurable parameters that are reflective of specific biological processes in living organisms. Diagnostic biomarkers point to disease type or severity and may support patient stratification or selection. Pharmacodynamic biomarkers are measured in clinical trials to indicate pharmacological responses to compounds.
- T cell-independent type 2 antigens
-
Polyvalent antigens that activate B cells by efficient crosslinking of the B cell receptor (BCR), without the need of T help. They differ from T cell-independent type 1 antigens, which are polyclonal B cell stimulants that activate B cells independently of BCR ligation.
Rights and permissions
About this article
Cite this article
Junt, T., Barchet, W. Translating nucleic acid-sensing pathways into therapies. Nat Rev Immunol 15, 529–544 (2015). https://doi.org/10.1038/nri3875
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3875
This article is cited by
-
First-in-Human Study of the Safety, Pharmacokinetics, and Pharmacodynamics of MHV370, a Dual Inhibitor of Toll-Like Receptors 7 and 8, in Healthy Adults
European Journal of Drug Metabolism and Pharmacokinetics (2023)
-
CXCL4 synergizes with TLR8 for TBK1-IRF5 activation, epigenomic remodeling and inflammatory response in human monocytes
Nature Communications (2022)
-
cGAS–STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells
Scientific Reports (2021)
-
The IRENA lncRNA converts chemotherapy-polarized tumor-suppressing macrophages to tumor-promoting phenotypes in breast cancer
Nature Cancer (2021)
-
Hepatitis B: a new weapon against an old enemy
Nature Medicine (2021)