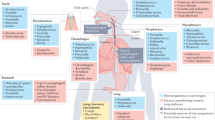
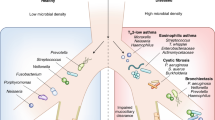
Abstract
Until recently, the airways were thought to be sterile unless infected; however, a shift towards molecular methods for the quantification and sequencing of bacterial DNA has revealed that the airways harbour a unique steady-state microbiota. This paradigm shift is changing the way that respiratory research is approached, with a clear need now to consider the effects of host–microorganism interactions in both healthy and diseased lungs. We propose that akin to recent discoveries in intestinal research, dysbiosis of the airway microbiota could underlie susceptibility to, and progression and chronicity of lung disease. In this Opinion article, we summarize current knowledge of the airway microbiota and outline how host–microorganism interactions in the lungs and other tissues might influence respiratory health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Erb-Downward, J. R. et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 6, e16384 (2011).
Hilty, M. et al. Disordered microbial communities in asthmatic airways. PLoS ONE 5, e8578 (2010).
Charlson, E. S. et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 184, 957–963 (2011).
Sze, M. A. et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 185, 1073–1080 (2012).
van der Gast, C. J. et al. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 5, 780–791 (2011).
Charlson, E. S. et al. Assessing bacterial populations in the lung by replicate analysis of samples from the upper and lower respiratory tracts. PLoS ONE 7, e42786 (2012).
Salter, S. Reagent contamination can critically impact sequence-based microbiome analyses. BioRxiv http://dx.doi.org/10.1101/007187 (2014).
Grice, E. A. & Segre, J. A. The skin microbiome. Nature Rev. Microbiol. 9, 244–253 (2011).
Frank, D. N. et al. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 5, e10598 (2010).
Lazarevic, V. et al. Metagenomic study of the oral microbiota by Illumina high-throughput sequencing. J. Microbiol. Methods 79, 266–271 (2009).
Kim, T. K. et al. Heterogeneity of vaginal microbial communities within individuals. J. Clin. Microbiol. 47, 1181–1189 (2009).
Maldonado-Contreras, A. et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 5, 574–579 (2011).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nature Rev. Genet. 13, 260–270 (2012).
Charlson, E. S. et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am. J. Respir. Crit. Care Med. 186, 536–545 (2012).
Madan, J. C. et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio 3, e00251-12 (2012).
Scupham, A. J. et al. Abundant and diverse fungal microbiota in the murine intestine. Appl. Environ. Microbiol. 72, 793–801 (2006).
Hoffmann, C. et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE 8, e66019 (2013).
Ghannoum, M. A. et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6, e1000713 (2010).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013).
Drell, T. et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 8, e54379 (2013).
Underhill, D. M. & Iliev, I. D. The mycobiota: interactions between commensal fungi and the host immune system. Nature Rev. Immunol. 14, 405–416 (2014).
Dollive, S. et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS ONE 8, e71806 (2013).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Ott, S. J. et al. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand. J. Gastroenterol. 43, 831–841 (2008).
Zhang, E. et al. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol. Immunol. 55, 625–632 (2011).
Noverr, M. C., Falkowski, N. R., McDonald, R. A., McKenzie, A. N. & Huffnagle, G. B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Erb Downward, J. R., Falkowski, N. R., Mason, K. L., Muraglia, R. & Huffnagle, G. B. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci. Rep. 3, 2191 (2013).
Wylie, K. M., Weinstock, G. M. & Storch, G. A. Emerging view of the human virome. Transl. Res. 160, 283–290 (2012).
Breitbart, M. & Rohwer, F. Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing. Biotechniques 39, 729–736 (2005).
Abeles, S. R. et al. Human oral viruses are personal, persistent and gender-consistent. ISME J. 8, 1753–1767 (2014).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Mallia, P. et al. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr. Pharm. Des. 13, 73–97 (2007).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 (2014).
Gollwitzer, E. S. et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nature Med. 20, 642–647 (2014).
Deshmukh, H. S. et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nature Med. 20, 524–530 (2014).
Lohmann, P. et al. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr. Res. 76, 294–301 (2014).
Gillilland, M. G. 3rd et al. Ecological succession of bacterial communities during conventionalization of germ-free mice. Appl. Environ. Microbiol. 78, 2359–2366 (2012).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Med. 20, 159–166 (2014).
Gollwitzer, E. S. & Marsland, B. J. Microbiota abnormalities in inflammatory airway diseases — Potential for therapy. Pharmacol. Ther. 141, 32–39 (2014).
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011).
Fanaro, S., Chierici, R., Guerrini, P. & Vigi, V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. Suppl. 91, 48–55 (2003).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Woodmansey, E. J. Intestinal bacteria and ageing. J. Appl. Microbiol. 102, 1178–1186 (2007).
Abrahamsson, T. R. et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 44, 842–850 (2014).
Power, S. E., O'Toole, P. W., Stanton, C., Ross, R. P. & Fitzgerald, G. F. Intestinal microbiota, diet and health. Br. J. Nutr. 111, 387–402 (2014).
Stevens, V., Dumyati, G., Fine, L. S., Fisher, S. G. & van Wijngaarden, E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin. Infect. Dis. 53, 42–48 (2011).
Sullivan, A., Edlund, C. & Nord, C. E. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1, 101–114 (2001).
Jernberg, C., Lofmark, S., Edlund, C. & Jansson, J. K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1, 56–66 (2007).
Dethlefsen, L. & Relman, D. A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4554–4561 (2011).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Russell, S. L. et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2014.06.027 (2014).
Brusselle, G. G. & Joos, G. Is there a role for macrolides in severe asthma? Curr. Opin. Pulm Med. 20, 95–102 (2014).
Pragman, A. A., Kim, H. B., Reilly, C. S., Wendt, C. & Isaacson, R. E. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 7, e47305 (2012).
Goleva, E. et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 188, 1193–1201 (2013).
Habibzay, M., Weiss, G. & Hussell, T. Bacterial superinfection following lung inflammatory disorders. Future Microbiol. 8, 247–256 (2013).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Goulding, J. et al. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J. Infect. Dis. 204, 1086–1094 (2011).
Mallia, P. et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186, 1117–1124 (2012).
Molyneaux, P. L. et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 188, 1224–1231 (2013).
Dicksved, J. et al. Molecular fingerprinting of the fecal microbiota of children raised according to different lifestyles. Appl. Environ. Microbiol. 73, 2284–2289 (2007).
von Mutius, E. & Vercelli, D. Farm living: effects on childhood asthma and allergy. Nature Rev. Immunol. 10, 861–868 (2010).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 15, 382–392 (2014).
Michail, S. et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 18, 1799–1808 (2012).
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22, 850–859 (2012).
Fahlen, A., Engstrand, L., Baker, B. S., Powles, A. & Fry, L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch. Dermatol. Res. 304, 15–22 (2012).
Huang, Y. J. & Lynch, S. V. The emerging relationship between the airway microbiota and chronic respiratory disease: clinical implications. Expert Rev. Respir. Med. 5, 809–821 (2011).
Dickson, R. P., Martinez, F. J. & Huffnagle, G. B. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 384, 691–702 (2014).
Sze, M. A., Hogg, J. C. & Sin, D. D. Bacterial microbiome of lungs in COPD. Int. J. Chron. Obstruct Pulmon Dis. 9, 229–238 (2014).
Craven, M. et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS ONE 7, e41594 (2012).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Herbst, T. et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 184, 198–205 (2011).
Hagner, S. et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy 68, 322–329 (2013).
Nembrini, C. et al. Bacterial-induced protection against allergic inflammation through a multicomponent immunoregulatory mechanism. Thorax 66, 755–763 (2011).
Thavagnanam, S., Fleming, J., Bromley, A., Shields, M. D. & Cardwell, C. R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 38, 629–633 (2008).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
von Mutius, E. Environmental factors influencing the development and progression of pediatric asthma. J. Allergy Clin. Immunol. 109, S525–S532 (2002).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Cahenzli, J., Koller, Y., Wyss, M., Geuking, M. B. & McCoy, K. D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570 (2013).
West, J. B. Regional differences in the lung. Chest 74, 426–437 (1978).
Ingenito, E. P. et al. Indirect assessment of mucosal surface temperatures in the airways: theory and tests. J. Appl. Physiol. 63, 2075–2083 (1987).
Duncan, S. H., Louis, P., Thomson, J. M. & Flint, H. J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 11, 2112–2122 (2009).
Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522–530 (2001).
Wolak, J. E., Esther, C. R. Jr & O'Connell, T. M. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers 14, 55–60 (2009).
Palmer, K. L., Aye, L. M. & Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087 (2007).
Moreau-Marquis, S., Stanton, B. A. & O'Toole, G. A. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol. Ther. 21, 595–599 (2008).
Spor, A., Koren, O. & Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nature Rev. Microbiol. 9, 279–290 (2011).
Abeles, S. R. & Pride, D. T. Molecular bases and role of viruses in the human microbiome. J. Mol. Biol. http://dx.doi.org/10.1016/j.jmb.2014.07.002 (2014).
Acknowledgements
This work was supported by Swiss National Science Foundation grant 310030_146983 (awarded to B.J.M.). B.J.M. is part of the European Cooperation in Science and Technology (COST) action BM1201, which is entitled “Developmental Origins of Chronic Lung Disease”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Constituents of the airway microbiota in health and disease. (PDF 613 kb)
Glossary
- Bronchoalveolar lavage fluid
-
Fluid containing bronchoalveolar cells that is obtained by infusing and extracting saline during bronchoscopy.
- Chronic obstructive pulmonary disease
-
(COPD). A chronic lung disorder that is particularly associated with cigarette smoking and is characterized by the presence of emphysema and chronic bronchitis.
- Cystic fibrosis
-
A genetic disorder caused by a mutation in the cystic fibrosis transmembrane conductance regulator that leads to recurrent respiratory infections and a progressive loss of lung function.
- Short-chain fatty acids
-
(SCFAs). Fatty acids with aliphatic tails of less than six carbons in length that are produced during bacterial fermentation of dietary fibres.
- Systemic metabolome
-
The complete set of small-molecule chemicals (metabolites) found within the bloodstream.
Rights and permissions
About this article
Cite this article
Marsland, B., Gollwitzer, E. Host–microorganism interactions in lung diseases. Nat Rev Immunol 14, 827–835 (2014). https://doi.org/10.1038/nri3769
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3769
This article is cited by
-
Lung microbiome: new insights into the pathogenesis of respiratory diseases
Signal Transduction and Targeted Therapy (2024)
-
Responsiveness to pulmonary rehabilitation in COPD is associated with changes in microbiota
Respiratory Research (2023)
-
Metabolome and microbiome multi-omics integration from a murine lung inflammation model of bronchopulmonary dysplasia
Pediatric Research (2022)
-
Effect of electronic cigarette and tobacco smoking on the human saliva microbial community
Brazilian Journal of Microbiology (2022)
-
A prevalent and culturable microbiota links ecological balance to clinical stability of the human lung after transplantation
Nature Communications (2021)