Key Points
-
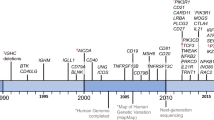
Primary antibody deficiencies (PADs) are the most common type of primary immunodeficiency and arise either alone or in combination with immunodeficiencies affecting other aspects of immunity against pathogens.
-
PADs are not solely due to B cell-intrinsic defects; they can also result from impairments in other cell lineages (especially T cells, but also innate immune cells). These observations emphasize the fact that B cell responses are sustained by both innate and adaptive immune signals.
-
Many PADs that result from B cell-intrinsic defects have now been characterized. These PADs variously involve all aspects of B cell biology, from B cell differentiation to B cell migration, survival and activation, and their characterization has helped to identify key molecules in B cell function.
-
B cell-extrinsic defects that result in PADs include defects in T cells, and particularly in T follicular helper cells, thus providing evidence for the essential role of these cells in antibody production. Some recently described defects in innate immune cells highlight the role of pattern-recognition receptors and T cell-independent antibody responses.
-
Depending on their underlying cause, PADs have been associated with various pathologies, including susceptibility to microbial infections, autoinflammatory and autoimmune diseases and some types of cancer.
Abstract
Primary antibody deficiencies (PADs) are the most common inherited immunodeficiencies in humans. The use of novel approaches, such as whole-exome sequencing and mouse genetic engineering, has helped to identify new genes that are involved in the pathogenesis of PADs and has enabled the characterization of the molecular pathways that are involved in B cell development and function. Here, we review the different PADs in terms of their known or putative mechanisms, which can be B cell intrinsic, B cell extrinsic or not defined so far. We also describe the clinical manifestations (including susceptibility to infections, autoimmunity and cancer) that have been associated with the various PADs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Conley, M. E. et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu. Rev. Immunol. 27, 199–227 (2009).
Berkowska, M. A., van der Burg, M., van Dongen, J. J. & van Zelm, M. C. Checkpoints of B cell differentiation: visualizing Ig-centric processes. Ann. NY Acad. Sci. 1246, 11–25 (2011).
Tangye, S. G., Deenick, E. K., Palendira, U. & Ma, C. S. T cell-B cell interactions in primary immunodeficiencies. Ann. NY Acad. Sci. 1250, 1–13 (2012).
Durandy, A., Revy, P. & Fischer, A. Human models of inherited immunoglobulin class switch recombination and somatic hypermutation defects (hyper-IgM syndromes). Adv. Immunol. 82, 295–330 (2004).
Meffre, E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann. NY Acad. Sci. 1246, 1–10 (2011).
Salzer, U. et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood 113, 1967–1976 (2009).
Conley, M. E. et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J. Exp. Med. 209, 463–470 (2012).
Vetrie, D. et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361, 226–233 (1993). This paper provided the first description of a gene defect causative of an inherited immune deficiency.
Fruman, D. A. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr. Opin. Immunol. 16, 314–320 (2004).
Lopez-Granados, E., Perez de Diego, R., Ferreira Cerdan, A., Fontan Casariego, G. & Garcia Rodriguez, M. C. A genotype-phenotype correlation study in a group of 54 patients with X-linked agammaglobulinemia. J. Allergy Clin. Immunol. 116, 690–697 (2005).
Bykowsky, M. J. et al. Discordant phenotype in siblings with X-linked agammaglobulinemia. Am. J. Hum. Genet. 58, 477–483 (1996).
Wood, P. M. et al. A mutation in Bruton's tyrosine kinase as a cause of selective anti-polysaccharide antibody deficiency. J. Pediatr. 139, 148–151 (2001).
Hernandez, P. A. et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nature Genet. 34, 70–74 (2003).
Derry, J. M., Ochs, H. D. & Francke, U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 78, 635–644 (1994).
Westerberg, L. et al. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood 105, 1144–1152 (2005).
Westerberg, L. S. et al. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood 119, 3966–3974 (2012). This study precisely characterizes the role of WASP in B cell development and function.
Becker-Herman, S. et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 208, 2033–2042 (2011).
Lanzi, G. et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J. Exp. Med. 209, 29–34 (2012).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Randall, K. L. et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nature Immunol. 10, 1283–1291 (2009). References 19 and 20 simultaneously provided the first description of the role of DOCK8 in B cells and T cell immunity.
Jabara, H. H. et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nature Immunol. 13, 612–620 (2012).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Nehme, N. T. et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012).
Abdollahpour, H. et al. The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012). References 23 and 24 simultaneously provided the first description of the role of MST1 in B cell and T cell immunity.
Warnatz, K. et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc. Natl Acad. Sci. USA 106, 13945–13950 (2009).
Wang, H. Y. et al. Antibody deficiency associated with an inherited autosomal dominant mutation in TWEAK. Proc. Natl Acad. Sci. USA 110, 5127–5132 (2013).
van Zelm, M. C. et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 354, 1901–1912 (2006). This paper provides the first description of an inherited hypogammaglobulinaemia caused by defective BCR co-stimulatory molecules.
van Zelm, M. C. et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Invest. 120, 1265–1274 (2010).
Thiel, J. et al. Genetic CD21 deficiency is associated with hypogammaglobulinemia. J. Allergy Clin. Immunol. 129, 801–810.e6 (2012).
Kuijpers, T. W. et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J. Clin. Invest. 120, 214–222 (2010).
Xu, G. L. et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402, 187–191 (1999).
de Greef, J. C. et al. Mutations in ZBTB24 are associated with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2. Am. J. Hum. Genet. 88, 796–804 (2011).
Blanco-Betancourt, C. E. et al. Defective B-cell-negative selection and terminal differentiation in the ICF syndrome. Blood 103, 2683–2690 (2004).
Picard, C. et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 360, 1971–1980 (2009).
Feske, S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006).
Stepensky, P. et al. Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects. J. Allergy Clin. Immunol. 131, 477–485.e1 (2013).
Snow, A. L. et al. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J. Exp. Med. 209, 2247–2261 (2012). References 36 and 37 report that deficiency in CARD11, a key protein in the canonical NF-κB pathway, leads to a PAD owing to both intrinsic and extrinsic B cell defects.
Zonana, J. et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-γ (NEMO). Am. J. Hum. Genet. 67, 6 (2000).
Hanson, E. P. et al. Hypomorphic nuclear factor-κB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J. Allergy Clin. Immunol. 122, 1169–1177.e16 (2008).
Pachlopnik Schmid, J. et al. Polymerase epsilon1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature ('FILS syndrome'). J. Exp. Med. 209, 2323–2330 (2012).
Courtois, G. et al. A hypermorphic IκBα mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J. Clin. Invest. 112, 1108–1115 (2003).
Ohnishi, H. et al. A rapid screening method to detect autosomal-dominant ectodermal dysplasia with immune deficiency syndrome. J. Allergy Clin. Immunol. 129, 578–580 (2012).
Victoratos, P. et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity 24, 65–77 (2006).
Boisson, B. et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nature Immunol. 13, 1178–1186 (2012).
Alangari, A. et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J. Allergy Clin. Immunol. 130, 481–488.e2 (2012).
Lopez-Herrera, G. et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am. J. Hum. Genet. 90, 986–1001 (2012).
Ferrari, S. et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc. Natl Acad. Sci. USA 98, 12614–12619 (2001).
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000). This study provides a description of AID as a key molecule in class-switch recombination and somatic hypermutation in humans.
Imai, K. et al. Analysis of class switch recombination and somatic hypermutation in patients affected with autosomal dominant hyper-IgM syndrome type 2. Clin. Immunol. 115, 277–285 (2005).
Ta, V. T. et al. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nature Immunol. 4, 843–848 (2003).
Imai, K. et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nature Immunol. 4, 1023–1028 (2003). The description of a new immunodeficiency in this paper provides strong evidence for a DNA-editing activity of AID in humans.
Du, L. et al. Cernunnos influences human immunoglobulin class switch recombination and may be associated with B cell lymphomagenesis. J. Exp. Med. 209, 291–305 (2012).
Stewart, G. S. et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434 (2009).
Peron, S. et al. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J. Exp. Med. 205, 2465–2472 (2008). This paper provided the first description in humans that mismatch repair enzymes are involved in class-switch recombination.
Gardes, P. et al. Human MSH6 deficiency is associated with impaired antibody maturation. J. Immunol. 188, 2023–2029 (2012).
Avery, D. T. et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207, 155–171 (2010).
Kotlarz, D. et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J. Exp. Med. 210, 433–443 (2013).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). This paper provides a description of IL-21 as a key cytokine in antibody production.
Recher, M. et al. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118, 6824–6835 (2011).
Hacein-Bey-Abina, S. et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 363, 355–364 (2010).
Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998).
Dadi, H. K., Simon, A. J. & Roifman, C. M. Effect of CD3δ deficiency on maturation of α/β and γ/δ T-cell lineages in severe combined immunodeficiency. N. Engl. J. Med. 349, 1821–1828 (2003).
de Saint Basile, G. et al. Severe combined immunodeficiency caused by deficiency in either the δ or the ε subunit of CD3. J. Clin. Invest. 114, 1512–1517 (2004).
Rieux-Laucat, F. et al. Inherited and somatic CD3ζ mutations in a patient with T-cell deficiency. N. Engl. J. Med. 354, 1913–1921 (2006).
Greenberg, F. et al. Familial DiGeorge syndrome and associated partial monosomy of chromosome 22. Hum. Genet. 65, 317–319 (1984).
Elder, M. E. et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science 264, 1596–1599 (1994).
Huck, K. et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J. Clin. Invest. 119, 1350–1358 (2009).
Hauck, F. et al. Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency. J. Allergy Clin. Immunol. 130, 1144–1152 e1111 (2012).
Lisowska-Grospierre, B. et al. A defect in the regulation of major histocompatibility complex class II gene expression in human HLA-DR negative lymphocytes from patients with combined immunodeficiency syndrome. J. Clin. Invest. 76, 381–385 (1985).
Ma, C. S., Deenick, E. K., Batten, M. & Tangye, S. G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 209, 1241–1253 (2012).
Warnatz, K. et al. Human ICOS-deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 107, 3045–3052 (2005).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177, 4927–4932 (2006). This study provided the first description of a PAD caused by defective T FH cell generation and germinal centre reactions.
Korthauer, U. et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature 361, 539–541 (1993). This paper provided the first description of the role of CD40L in antibody maturation.
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunol. 3, 822–829 (2002).
Weller, S. et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood 120, 4992–5001 (2012).
Weller, S. et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl Acad. Sci. USA 98, 1166–1170 (2001).
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L. & Germain, R. N. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008).
Sayos, J. et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
van Montfrans, J. M. et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J. Allergy Clin. Immunol. 129, 787–793.e6 (2012).
Salzer, E. et al. Combined immunodeficiency with life-threatening EBV-associatedlymphoproliferative disorder in patients lacking functional CD27. Haematologica 98, 473–478 (2012).
Ma, C. S. et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 119, 3997–4008 (2012).
Puga, I. et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nature Immunol. 13, 170–180 (2011).
Moir, S. et al. Humans with chronic granulomatous disease maintain humoral immunologic memory despite low frequencies of circulating memory B cells. Blood 120, 4850–4858 (2012).
Chapel, H. et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J. Allergy Clin. Immunol. 130, 1197–1198.e99 (2012).
Resnick, E. S., Moshier, E. L., Godbold, J. H. & Cunningham-Rundles, C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 119, 1650–1657 (2012).
Orange, J. S. et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J. Allergy Clin. Immunol. 127, 1360–1367 e1366 (2011).
Brandtzaeg, P. & Johansen, F. E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 206, 32–63 (2005).
Salzer, U. et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nature Genet. 37, 820–828 (2005).
Castigli, E. et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nature Genet. 37, 829–834 (2005).
Rioux, J. D. et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc. Natl Acad. Sci. USA 106, 18680–18685 (2009).
Lefranc, M. P., Lefranc, G. & Rabbitts, T. H. Inherited deletion of immunoglobulin heavy chain constant region genes in normal human individuals. Nature 300, 760–762 (1982).
Kruetzmann, S. et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197, 939–945 (2003).
Micol, R. et al. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J. Allergy Clin. Immunol. 129, 770–777 (2012).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Yoshizaki, A. et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268 (2012).
Shlomchik, M. J., Marshak-Rothstein, A., Wolfowicz, C. B., Rothstein, T. L. & Weigert, M. G. The role of clonal selection and somatic mutation in autoimmunity. Nature 328, 805–811 (1987).
Durandy, A., Cantaert, T., Kracker, S. & Meffre, E. Potential roles of activation-induced cytidine deaminase in promotion or prevention of autoimmunity in humans. Autoimmunity 46, 148–156 (2012).
Hase, K. et al. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS ONE 3, e3033 (2008).
Rakhmanov, M. et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc. Natl Acad. Sci. USA 106, 13451–13456 (2009).
Isnardi, I. et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood 115, 5026–5036 (2010).
De Ravin, S. S. et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 116, 1263–1271 (2010).
Schuetz, C. et al. An immunodeficiency disease with RAG mutations and granulomas. N. Engl. J. Med. 358, 2030–2038 (2008).
Ombrello, M. J. et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 366, 330–338 (2012).
Zhou, Q. et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 (2012).
Feske, S., Picard, C. & Fischer, A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clin. Immunol. 135, 169–182 (2010).
Moratto, D. et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott–Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood 118, 1675–1684 (2011).
Hayward, A. R. et al. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 158, 977–983 (1997).
Jain, A. et al. Partial immune reconstitution of X-linked hyper IgM syndrome with recombinant CD40 ligand. Blood 118, 3811–3817 (2011).
Aiuti, A. et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 360, 447–458 (2009).
Boztug, K. et al. Stem-cell gene therapy for the Wiskott–Aldrich syndrome. N. Engl. J. Med. 363, 1918–1927 (2010).
Kerns, H. M. et al. B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood 115, 2146–2155 (2010).
Dissing, J. & Knudsen, B. Adenosine-deaminase deficiency and combined immunodeficiency syndrome. Lancet 2, 1316 (1972).
Giblett, E. R., Anderson, J. E., Cohen, F., Pollara, B. & Meuwissen, H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet 2, 1067–1069 (1972).
Lagresle-Peyrou, C. et al. Human adenylate kinase 2 deficiency causes a profound hematopoietic defect associated with sensorineural deafness. Nature Genet. 41, 106–111 (2009).
Pannicke, U. et al. Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2. Nature Genet. 41, 101–105 (2009).
Nelson, N. D. & Bertuch, A. A. Dyskeratosis congenita as a disorder of telomere maintenance. Mutat. Res. 730, 43–51 (2012).
Dickinson, R. E. et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 118, 2656–2658 (2011).
Moshous, D. et al. Partial T and B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. J. Clin. Invest. 111, 381–387 (2003).
Schwarz, K. et al. RAG mutations in human B cell-negative SCID. Science 274, 97–99 (1996).
Acknowledgements
The authors apologize to all colleagues whose work could not be cited owing to length restrictions. The authors thank C. Picard for critical reading of the manuscript. This work was funded by grants from Institut National de la Santé et de la Recherche Médicale, the European Union's 7th RTD Framework Programme (EURO-PADnet grant number 201549 and ERC PIDIMMUNE grant number 249816), Association Contre Le Cancer and ANR Blanc 2010-CSRD. S.K. is a Centre National de la Recherche Scientifique (CNRS) researcher.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
PADs related to B cell-intrinsic defects (PDF 125 kb)
Supplementary information S2 (table)
PADs related to B cell-extrinsic defects (PDF 116 kb)
Glossary
- Pre-B cell receptor
-
(Pre-BCR). A receptor that is formed at the surface of pre-B cells when rearranged immunoglobulin heavy chains pair with surrogate immunoglobulin light chains; the pre-BCR is associated with signalling heterodimers of Igα and Igβ. Signalling through the pre-BCR occurs in the absence of known ligands and is a crucial event in B cell development.
- Pre-B cells
-
Haematopoietic cells that appear in the bone marrow early during B cell development, downstream of the CD34+ pro-B cell precursor. At the pre-B1 stage, the cells still express CD34 but acquire the B cell-specific marker CD19. Pre-B2 cells are characterized by complete immunoglobulin heavy chain rearrangement in the absence of immunoglobulin light chain rearrangement. They express the pre-B cell receptor (which comprises a pseudo-light chain and the -heavy chain), CD19 and cytoplasmic IgM.
- V(D)J recombination
-
A site-specific recombination process (targeting recombination signal sequences) that takes place in primary lymphoid tissues and stochastically combines the different regions of the T cell and B cell receptors (variable (V), diversity (D) and joining (J) regions) in pre-T and pre-B cell precursors. The lymphoid-specific enzymes RAG1 and RAG2 and the non-lymphoid-specific non-homologous end joining DNA repair complex (which includes DNA protein kinase, Ku70–Ku80, Artemis, XRCC4, DNA ligase 4 and Cernunnos) control this process.
- Polysaccharide-specific antibodies
-
Antibodies that are secreted during T cell-independent antibody responses (especially by marginal zone B cells).
- Marginal zone
-
An anatomical site located at the interface between the red pulp and the lymphoid white pulp of the spleen, in which marginal zone B cells are rapidly recruited into early, adaptive immune responses in a T cell-independent manner. Marginal zone B cells produce IgM as the first line of defence against blood-borne antigens.
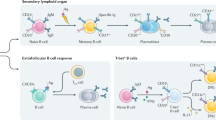
- Germinal centre
-
Within secondary lymphoid tissues, B cells exposed to migration signals (through CXCL13–CXCR5) enter the follicles and, following interaction with cognate T cells, undergo vigorous proliferation and form germinal centres. B cells undergo class-switch recombination and somatic hypermutation in these germinal centres.
- T cell-dependent antibody response
-
Antibody response to protein antigens that require T cell help. This response mostly occurs in the germinal centre in secondary lymphoid organs, via CD40L–CD40 interactions.
- T cell-independent antibody response
-
Antibody response to polymeric antigens, such as polysaccharides and lipids, that do not require T cell help.
- Class-switch recombination
-
(CSR). Region-specific DNA recombination between two different switch regions located upstream of the constant (C) regions in the immunoglobulin locus, with excision of the intervening DNA. Replacement of the Cμ region by a downstream C region from another class of immunoglobulin results in the production of antibodies of different isotypes (IgG, IgA and IgE).
- Somatic hypermutation
-
(SHM). SHM introduces mutations into the immunoglobulin variable regions and is a major component of affinity maturation, providing a basis for the selection and proliferation of B cells expressing a B cell receptor with a high affinity for the antigen.
- T follicular helper cell
-
(TFH cell). A germinal centre T helper cell that expresses the chemokine receptor CXCR5 and the co-stimulatory molecules CD40L and ICOS, but only low levels of CCR7. TFH cells are essential for class-switch recombination and somatic hypermutation in B cells. They secrete cytokines (especially interleukin-21, which acts in a paracrine and autocrine manner).
- Activation-induced cytidine deaminase
-
(AID). A key enzyme that induces somatic hypermutation and class-switch recombination by deaminating cytosine bases to uracil bases in single-stranded DNA in the variable and switch regions of the immunoglobulin locus.
- Uracil N-glycosylase
-
(UNG). A base excision repair enzyme that recognizes and removes uracils (including those induced by AID) from within DNA.
- Mismatch repair
-
(MMR). A repair pathway that recognizes and corrects mismatched base pairs (typically those that arise from errors of chromosomal DNA replication). There are two main types of MMR components: MutS homologues (MSH1–MSH6) and MutL homologues (PMS2, MLH1 and PMS1).
- B cell tolerance
-
B cell tolerance is controlled by two checkpoints: central B cell tolerance is achieved in the bone marrow, through the removal of immature B cells that express polyreactive antibodies, whereas peripheral B cell tolerance mechanisms operate at the transition between immature and mature naive B cells and counterselect self-reactive B cells that may have encountered peripheral autoantigens that are not expressed in the bone marrow. Disruption of B cell tolerance leads to autoimmunity.
Rights and permissions
About this article
Cite this article
Durandy, A., Kracker, S. & Fischer, A. Primary antibody deficiencies. Nat Rev Immunol 13, 519–533 (2013). https://doi.org/10.1038/nri3466
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3466
This article is cited by
-
Role of IL-27 in Epstein–Barr virus infection revealed by IL-27RA deficiency
Nature (2024)
-
Clinical Validation of a Primary Antibody Deficiency Screening Algorithm for Primary Care
Journal of Clinical Immunology (2023)
-
Is there a role for microbiome-based approach in common variable immunodeficiency?
Clinical and Experimental Medicine (2023)
-
Screening for Antibody Deficiencies in Adults by Serum Electrophoresis and Calculated Globin
Journal of Clinical Immunology (2023)
-
The TRIM37 variants in Mulibrey nanism patients paralyze follicular helper T cell differentiation
Cell Discovery (2023)