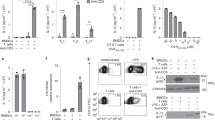
Key Points
-
Toll-like receptors (TLRs) are members of the pattern recognition receptor (PRR) family. They sense pathogen-derived molecules termed pathogen-associated molecular patterns (PAMPs), as well as endogenous molecules termed damage-associated molecular patterns (DAMPs) that are released from dead and dying cells. Activation of TLR signalling pathways in innate immune cells, such as dendritic cells (DCs), drives adaptive immunity by enhancing the ability of DCs to act as antigen-presenting cells and promoting the production of pro-inflammatory cytokines that direct the induction of different T cell subtypes.
-
Autoimmune diseases can develop as a result of a breakdown in immune tolerance that leads to the activation of autoantigen-specific T cells. Self-reactive T cells that secrete interleukin-17 (TH17 cells), interferon-γ (TH1 cells) or both cytokines mediate inflammatory pathology in many autoimmune diseases.
-
Infectious pathogens and the gut microbiota have been implicated in precipitating or exacerbating autoimmune diseases in humans. Studies in mouse models and with tissues from patients with autoimmune diseases have suggested that PAMPs may promote innate and consequently adaptive immune responses that promote inflammation and tissue damage.
-
The release of endogenous DAMPs from host cells that have been killed as a result of damage or infection with pathogens can activate innate immune responses, driving sterile inflammation that initiates or exacerbates pathology in autoimmune diseases.
-
Inhibition of agonist binding to TLRs or downstream signalling pathways is a promising approach for the development of therapies for inflammatory and autoimmune diseases.
Abstract
Autoimmune disease can develop as a result of a breakdown in immunological tolerance, leading to the activation of self-reactive T cells. There is an established link between infection and human autoimmune diseases. Furthermore, experimental autoimmune diseases can be induced by autoantigens that are administered together with complete Freund's adjuvant, which contains killed Mycobacterium tuberculosis; in some cases, these bacteria can be replaced by individual pathogen-associated molecular patterns (PAMPs). Exogenous PAMPs and endogenous danger signals from necrotic cells bind to pattern recognition receptors (including Toll-like receptors) and activate signalling pathways in innate immune cells and in T cells. This leads to pro-inflammatory cytokine production and T cell activation, which are now considered to be major factors in the development of autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Mills, K. H. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 38, 2636–2649 (2008).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunol. 11, 7–13 (2010).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Hennessy, E. J., Parker, A. E. & O'Neill, L. A. Targeting Toll-like receptors: emerging therapeutics? Nature Rev. Drug Discov. 9, 293–307 (2010).
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009). The first report that γδ T cells promote autoimmune inflammation by providing a source of innate IL-17 and IL-21.
van Beelen, A. J. et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 (2007). The first report that sensing of PAMPs through NLRs promotes the development of human T H 17 cells.
Lalor, S. J. et al. Caspase-1-processed cytokines IL-1β and IL-18 promote IL-17 production by γδ and CD4 T cells that mediate autoimmunity. J. Immunol. 186, 5738–5748 (2011). This study defined a role for PAMP-induced IL-18 and IL-1 in driving IL-17 production by CD4+ and γδ T cells that are pathogenic in autoimmune diseases.
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). This study demonstrated that certain strains of commensal bacteria promote the induction of T H 17 cells in the intestine, suggesting that the microbiota may precipitate autoimmunity.
Prinz, M. et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116, 456–464 (2006). This study demonstrated that signalling through MYD88 and TLR9 is required to promote the innate cytokines that drive the induction of T H 17 cells in experimental autoimmunity.
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008). This study demonstrated that TLR4 may be an important drug target for rheumatoid arthritis; activation of TLR4 by the microbiota promoted the generation of T cells that were pathogenic in an arthritis model, and disease was blocked using a TLR4 antagonist.
Brereton, C. F., Sutton, C. E., Lalor, S. J., Lavelle, E. C. & Mills, K. H. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J. Immunol. 183, 1715–1723 (2009).
Jarnicki, A. G. et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J. Immunol. 180, 3797–3806 (2008).
Conroy, H., Marshall, N. A. & Mills, K. H. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene 27, 168–180 (2008).
Higgins, S. C. & Mills, K. H. TLR, NLR agonists, and other immune modulators as infectious disease vaccine adjuvants. Curr. Infect. Dis. Rep. 12, 4–12 (2010).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Sutton, C., Brereton, C., Keogh, B., Mills, K. H. & Lavelle, E. C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 6, 1133–1141 (2005).
Martin, B., Hirota, K., Cua, D. J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Rachitskaya, A. V. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 (2008).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Murphy, A. C., Lalor, S. J., Lynch, M. A. & Mills, K. H. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 641–651 (2010).
Park, Y., Park, S., Yoo, E., Kim, D. & Shin, H. Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann. NY Acad. Sci. 1037, 170–174 (2004).
Hong, J. et al. TLR2, TLR4 and TLR9 polymorphisms and Crohn's disease in a New Zealand Caucasian cohort. J. Gastroenterol. Hepatol. 22, 1760–1766 (2007).
van Heel, D. A. et al. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn's disease. Gut 54, 1553–1557 (2005).
Kamradt, T., Goggel, R. & Erb, K. J. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 26, 260–267 (2005).
Serafini, B. et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Buljevac, D. et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125, 952–960 (2002).
Correale, J., Fiol, M. & Gilmore, W. The risk of relapses in multiple sclerosis during systemic infections. Neurology 67, 652–659 (2006).
Saal, J. G. et al. Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol. Int. 12, 147–151 (1992).
Saal, J. G. et al. Synovial Epstein-Barr virus infection increases the risk of rheumatoid arthritis in individuals with the shared HLA-DR4 epitope. Arthritis Rheum. 42, 1485–1496 (1999).
Abraham, C. & Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 140, 1729–1737 (2011).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Atarashi, K. et al. ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812 (2008).
Feng, T., Wang, L., Schoeb, T. R., Elson, C. O. & Cong, Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 207, 1321–1332 (2010).
Herrmann, I. et al. Streptococcus pneumoniae infection aggravates experimental autoimmune encephalomyelitis via Toll-like receptor 2. Infect. Immun. 74, 4841–4848 (2006).
Schrijver, I. A., Melief, M. J., Tak, P. P., Hazenberg, M. P. & Laman, J. D. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 43, 2160–2168 (2000).
Klasen, I. S. et al. The presence of peptidoglycan–polysaccharide complexes in the bowel wall and the cellular responses to these complexes in Crohn's disease. Clin. Immunol. Immunopathol. 71, 303–308 (1994).
Visser, L. et al. Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates. Am. J. Pathol. 169, 1671–1685 (2006).
Schrijver, I. A. et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 124, 1544–1554 (2001).
Bsibsi, M., Ravid, R., Gveric, D. & van Noort, J. M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 61, 1013–1021 (2002).
Rajan, N. & Langtry, J. A. Generalized exacerbation of psoriasis associated with imiquimod cream treatment of superficial basal cell carcinomas. Clin. Exp. Dermatol. 31, 140–141 (2006).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Maizels, R. M. & Yazdanbakhsh, M. T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem. Immunol. Allergy 94, 112–123 (2008).
Strachan, D. P. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55, S2–S10 (2000).
Walsh, K. P., Brady, M. T., Finlay, C. M., Boon, L. & Mills, K. H. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J. Immunol. 183, 1577–1586 (2009).
Ochoa-Reparaz, J. et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010).
Seong, S. Y. & Matzinger, P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nature Rev. Immunol. 4, 469–478 (2004).
Oppenheim, J. J. & Yang, D. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17, 359–365 (2005).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Yanai, H. et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462, 99–103 (2009).
Li, J. et al. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J. Rheumatol. 37, 766–775 (2010).
Chen, C. J. et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Med. 13, 851–856 (2007).
Farez, M. F. et al. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nature Immunol. 10, 958–964 (2009).
Sacre, S. M. et al. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am. J. Pathol. 170, 518–525 (2007).
Hoffmann, M. H. et al. Nucleic acid-stimulated antigen-presenting cells trigger T cells to induce disease in a rat transfer model of inflammatory arthritis. J. Autoimmun. 36, 288–300 (2011).
Marta, M., Andersson, A., Isaksson, M., Kampe, O. & Lobell, A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 38, 565–575 (2008).
Reynolds, J. M. et al. Toll-like receptor 2 signaling in CD4+ T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32, 692–702 (2010).
Ichikawa, H. T., Williams, L. P. & Segal, B. M. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J. Immunol. 169, 2781–2787 (2002). This study demonstrated that innate immune cell activation and IL-12 induction through TLR9 induces T cells that are pathogenic in EAE.
Hall, J. A. et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 (2008).
Touil, T., Fitzgerald, D., Zhang, G. X., Rostami, A. & Gran, B. Cutting edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β. J. Immunol. 177, 7505–7509 (2006).
Onta, T. et al. Induction of acute arthritis in mice by peptidoglycan derived from Gram-positive bacteria and its possible role in cytokine production. Microbiol. Immunol. 37, 573–582 (1993).
Frasnelli, M. E., Tarussio, D., Chobaz-Peclat, V., Busso, N. & So, A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res. Ther. 7, R370–R379 (2005).
Ronaghy, A. et al. Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J. Immunol. 168, 51–56 (2002).
Abdollahi-Roodsaz, S. et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967 (2007).
Su, S. B. et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175, 6303–6310 (2005).
Kim, H. S. et al. Toll-like receptor 2 senses β-cell death and contributes to the initiation of autoimmune diabetes. Immunity 27, 321–333 (2007).
Summers, S. A. et al. Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum. 63, 1124–1135 (2011).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Evans, H. G., Suddason, T., Jackson, I., Taams, L. S. & Lord, G. M. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl Acad. Sci. USA 104, 17034–17039 (2007).
Higgins, S. C., Jarnicki, A. G., Lavelle, E. C. & Mills, K. H. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177, 7980–7989 (2006).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 8, 639–646 (2007).
LeibundGut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature Immunol. 8, 630–638 (2007).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Tigno-Aranjuez, J. T., Jaini, R., Tuohy, V. K., Lehmann, P. V. & Tary-Lehmann, M. Encephalitogenicity of complete Freund's adjuvant relative to CpG is linked to induction of Th17 cells. J. Immunol. 183, 5654–5661 (2009).
Waldner, H., Collins, M. & Kuchroo, V. K. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J. Clin. Invest. 113, 990–997 (2004). This study demonstrated that activation of APCs with a TLR9 agonist can promote self-reactive T cells that mediate autoimmunity in mice.
Veldhoen, M., Hocking, R. J., Flavell, R. A. & Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunol. 7, 1151–1156 (2006).
Hansen, B. S., Hussain, R. Z., Lovett-Racke, A. E., Thomas, J. A. & Racke, M. K. Multiple Toll-like receptor agonists act as potent adjuvants in the induction of autoimmunity. J. Neuroimmunol. 172, 94–103 (2006).
Peng, G. et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309, 1380–1384 (2005).
Pasare, C. & Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036 (2003).
Yang, Y., Huang, C. T., Huang, X. & Pardoll, D. M. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nature Immunol. 5, 508–515 (2004).
Kubo, T. et al. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J. Immunol. 173, 7249–7258 (2004).
den Haan, J. M., Kraal, G. & Bevan, M. J. Cutting edge: lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J. Immunol. 178, 5429–5433 (2007).
Imanishi, T. et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 178, 6715–6719 (2007).
Marsland, B. J. et al. TLR ligands act directly upon T cells to restore proliferation in the absence of protein kinase C-θ signaling and promote autoimmune myocarditis. J. Immunol. 178, 3466–3473 (2007).
Melzer, N., Meuth, S. G. & Wiendl, H. CD8+ T cells and neuronal damage: direct and collateral mechanisms of cytotoxicity and impaired electrical excitability. FASEB J. 23, 3659–3673 (2009).
Wong, C. K. et al. Activation profile of Toll-like receptors of peripheral blood lymphocytes in patients with systemic lupus erythematosus. Clin. Exp. Immunol. 159, 11–22 (2010).
Hornung, V. et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 (2002).
Cottalorda, A. et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur. J. Immunol. 36, 1684–1693 (2006).
Roark, C. L., Simonian, P. L., Fontenot, A. P., Born, W. K. & O'Brien, R. L. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 20, 353–357 (2008).
Caramalho, I. et al. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197, 403–411 (2003).
Crellin, N. K. et al. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 175, 8051–8059 (2005).
Sutmuller, R. P. et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116, 485–494 (2006).
Liu, H., Komai-Koma, M., Xu, D. & Liew, F. Y. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl Acad. Sci. USA 103, 7048–7053 (2006).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Fort, M. M. et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174, 6416–6423 (2005).
Ungaro, R. et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1167–G1179 (2009).
Mullarkey, M. et al. Inhibition of endotoxin response by E5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 304, 1093–1102 (2003).
Eisai Co., Ltd. Phase III study for severe sepsis treatment eritoran (E5564) does not meet primary endpoint. Eisai Co., Ltd.[online], (2011).
Arslan, F. et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic Toll-like receptor-2 and reduced by systemic administration of a novel anti-Toll-like receptor-2 antibody. Circulation 121, 80–90 (2010).
Lazzaro, B. P. & Rolff, J. Immunology. Danger, microbes, and homeostasis. Science 332, 43–44 (2011).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
US Food and Drug Administration. FDA briefing document: supplemental BLA 125319. FDA [online], (2011).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Vermeire, K. et al. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J. Immunol. 158, 5507–5513 (1997).
Krakowski, M. & Owens, T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26, 1641–1646 (1996).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Murphy, C. A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Kroenke, M. A., Carlson, T. J., Andjelkovic, A. V. & Segal, B. M. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205, 1535–1541 (2008).
Luger, D. et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008).
Panitch, H. S., Hirsch, R. L., Haley, A. S. & Johnson, K. P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1, 893–895 (1987).
Masters, S. L. et al. Regulation of interleukin-1β by interferon-γ is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 11, 640–646 (2010).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Fang, J. et al. The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Invest. Ophthalmol. Vis. Sci. 51, 3092–3099 (2010).
Yoshino, S., Sasatomi, E. & Ohsawa, M. Bacterial lipopolysaccharide acts as an adjuvant to induce autoimmune arthritis in mice. Immunology 99, 607–614 (2000).
Acknowledgements
Kingston Mills is supported by Science Foundation Ireland, The Irish Health Research Board and Enterprise Ireland. I am grateful to J. Fletcher, C. Sutton and R. Higgs for helpful discussions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Kingston Mills is a co-founder and shareholder in Opsona Therapeutics Ltd and TriMod Therapeutics Ltd, which are university spin-out companies involved in the development of immunotherapeutics.
Related links
Glossary
- Microorganism-associated molecular patterns
-
(MAMPs). Molecular patterns that are found in pathogens and commensal microorganisms but not in mammalian cells.
- Damage-associated molecular patterns
-
(DAMPs). Molecular patterns that are found in mammalian cells and are released as a result of cellular stress, cellular damage or non-physiological cell death. Examples include hyaluronate (which is released from the degraded stroma); HMGB1 (which is released from the nucleus); and ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins (which are released from the cytosol). Such DAMPs are thought to elicit local inflammatory reactions.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecular patterns that are found in pathogens but not in mammalian cells. Examples include terminally mannosylated and polymannosylated compounds (which bind the mannose receptor) and various microbial components, such as bacterial lipopolysaccharide, hypomethylated DNA, flagellin and double-stranded RNA (all of which bind Toll-like receptors).
- Sterile inflammation
-
Inflammation, characterized by leukocyte recruitment, that does not involve infection but is precipitated by the activation of innate immune cells by endogenous mediators (alarmins or DAMPs) that are released from host cells following tissue injury and necrotic cell death.
- NLRP3 inflammasome
-
(Nucleotide-binding oligomerization domain-, leucine rich repeat- and pyrin domain-containing 3 inflammasome). The NLRP proteins are a family of cytoplasmic proteins that can form high molecular weight signalling complexes, termed inflammasomes. The NLRP3 inflammasome contains the adaptor molecule ASC and recruits pro-caspase 1, which is then activated by autocatalytic cleavage. Active caspase 1 catalyses the cleavage of pro-IL-1β, pro-IL-18 and pro-IL-33, resulting in the secretion of biologically active forms of these cytokines. The NLRP3 inflammasome mediates innate immune responses to exogenous bacteria, environmental molecules (such as alum and asbestos) and endogenous molecules (such as ATP and amyloid-β).
- Lymphoid tissue inducer-like cells
-
Innate lymphoid cells that constitutively express CD4, RORγt, the IL-23 receptor, the aryl hydrocarbon receptor and CCR6, but not CD3, NK1.1, CD11b, GR1, CD11c or B220. These cells are found in the spleen and the lamina propria and are an early source of IL-17 and IL-22 in host defence and an important source of IL-22 in intestinal homeostasis.
- Specific pathogen free conditions
-
(SPF conditions). Vivarium conditions for rodents in which an increasing number of pathogens are excluded or eradicated from the colony. These animals are maintained in the absence of most of the known chronic and latent persistent pathogens. Although this enables better control of experimental conditions related to immunity and infection, it also sets apart such animal models from pathogen-exposed humans or non-human primates, whose immune systems are in constant contact with infectious agents.
Rights and permissions
About this article
Cite this article
Mills, K. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol 11, 807–822 (2011). https://doi.org/10.1038/nri3095
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3095
This article is cited by
-
p53 downregulates PD-L1 expression via miR-34a to inhibit the growth of triple-negative breast cancer cells: a potential clinical immunotherapeutic target
Molecular Biology Reports (2023)
-
Neuroprotective Effects of Sinomenine on Experimental Autoimmune Encephalomyelitis via Anti-Inflammatory and Nrf2-Dependent Anti-Oxidative Stress Activity
NeuroMolecular Medicine (2023)
-
IL-17A Facilitates Entry of Autoreactive T-Cells and Granulocytes into the CNS During EAE
NeuroMolecular Medicine (2023)
-
NDAMM: a numerical differentiation-based artificial macrophage model for anomaly detection
Applied Intelligence (2023)
-
Application and prospect of targeting innate immune sensors in the treatment of autoimmune diseases
Cell & Bioscience (2022)