Key Points
-
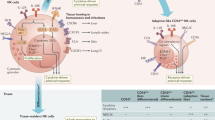
Dendritic cells (DCs) use distinct cell-specific receptors for binding to HIV that differentially affect both the fate of the virus and the subsequent effect of the virus on DCs.
-
The ability of conventional DCs (cDCs) to respond to HIV-1 is dysregulated. This may be due to a lack of Toll-like receptor (TLR)-mediated recognition of the virus; the modulation of internal signalling in cDCs by HIV-1 (such as through C-type lectins); or an indirect effect mediated by factors that are produced in the environment during HIV-1 infection. Such dysregulation may impede the ability of cDCs to stimulate adaptive immune responses to HIV-1.
-
Plasmacytoid DCs (pDCs) respond to HIV-1 through TLR7-mediated recognition of viral genomic RNA and produce type I interferons, which can limit viral replication but also contribute to bystander T cell death. pDCs further contribute to deleterious immunopathology during HIV-1 infection by secreting chemokines that attract uninfected T cells to the site of infection, and by inducing regulatory T cells, which can suppress the immune response to HIV-1.
-
Natural killer (NK) cells play an important immunoregulatory role in viral infection, including during HIV-1 infection, by secreting cytokines and by 'editing' DC function.
-
NK cells can also directly recognize HIV-1-infected cells using an array of different receptors, including activating receptors, killer cell immunoglobulin-like receptors and the CD16 receptor, which mediates antibody-dependent cell cytotoxicity.
-
Crosstalk between NK cells and DCs is crucial for optimizing the maturation of DCs and enhancing the ability of DCs to prime adaptive immune responses. However, HIV-1 infection interferes with this crosstalk.
Abstract
Dendritic cells (DCs) and natural killer (NK) cells have central roles in antiviral immunity by shaping the quality of the adaptive immune response to viruses and by mediating direct antiviral activity. HIV-1 infection is characterized by a severe dysregulation of the antiviral immune response that starts during early infection. This Review describes recent insights into how HIV-1 infection affects DC and NK cell function, and the roles of these innate immune cells in HIV-1 pathogenesis. The importance of understanding DC and NK cell crosstalk during HIV infection for the development of effective antiviral strategies is also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McMichael, A. J., Borrow, P., Tomaras, G. D., Goonetilleke, N. & Haynes, B. F. The immune response during acute HIV-1 infection: clues for vaccine development. Nature Rev. Immunol. 10, 11–23 (2010).
Liu, Y. J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Rescigno, M. & Borrow, P. The host-pathogen interaction: new themes from dendritic cell biology. Cell 106, 267–270 (2001).
Ferlazzo, G. et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl Acad. Sci. USA 101, 16606–16611 (2004).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Romagnani, C. et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+CD25hi T regulatory cells. Eur. J. Immunol. 35, 2452–2458 (2005).
Wu, L. & KewalRamani, V. N. Dendritic-cell interactions with HIV: infection and viral dissemination. Nature Rev. Immunol. 6, 859–868 (2006).
Ignatius, R. et al. The immunodeficiency virus coreceptor, Bonzo/STRL33/TYMSTR, is expressed by macaque and human skin- and blood-derived dendritic cells. AIDS Res. Hum. Retroviruses 16, 1055–1059 (2000).
Geijtenbeek, T. B. et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000).
Schmidt, B., Ashlock, B. M., Foster, H., Fujimura, S. H. & Levy, J. A. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology 343, 256–266 (2005).
Boggiano, C., Manel, N. & Littman, D. R. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J. Virol. 81, 2519–2523 (2007).
Sabatte, J. et al. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J. Virol. 81, 13723–13734 (2007).
Lambert, A. A., Gilbert, C., Richard, M., Beaulieu, A. D. & Tremblay, M. J. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 112, 1299–1307 (2008).
de Witte, L. et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Med. 13, 367–371 (2007).
Fahrbach, K. M. et al. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J. Virol. 81, 6858–6868 (2007).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Beignon, A. S. et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J. Clin. Invest. 115, 3265–3275 (2005). This study showed how HIV stimulates pDCs for type I IFN production through TLR7 signalling.
Meier, A. et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 81, 8180–8191 (2007).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Sabado, R. L. et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood 116, 3839–3852 (2010). This study examined the changes that occur in cDC and pDC frequency and function over the course of early HIV infection.
Smed-Sorensen, A. et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79, 8861–8869 (2005).
Manel, N. et al. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467, 214–217 (2010).
Geijtenbeek, T. B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Gringhuis, S. I. et al. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nature Immunol. 11, 419–426 (2010). This study showed that productive HIV infection in cDCs is induced by TLR8 signalling, which initiates viral RNA transcription, and DC-SIGN signalling, which promotes transcript elongation.
Deretic, V. Multiple regulatory and effector roles of autophagy in immunity. Curr. Opin. Immunol. 21, 53–62 (2009).
Blanchet, F. P. et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 32, 654–669 (2010).
Granelli-Piperno, A., Shimeliovich, I., Pack, M., Trumpfheller, C. & Steinman, R. M. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J. Immunol. 176, 991–998 (2006).
Granelli-Piperno, A., Golebiowska, A., Trumpfheller, C., Siegal, F. P. & Steinman, R. M. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl Acad. Sci. USA 101, 7669–7674 (2004).
Harman, A. N. et al. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood 114, 85–94 (2009).
Poulin, L. F. et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J. Exp. Med. 207, 1261–1271 (2010).
Donaghy, H., Gazzard, B., Gotch, F. & Patterson, S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101, 4505–4511 (2003).
Cameron, P. U. et al. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J. Virol. 81, 2297–2306 (2007).
Kodama, A. et al. Impairment of in vitro generation of monocyte-derived human dendritic cells by inactivated human immunodeficiency virus-1: involvement of type I interferon produced from plasmacytoid dendritc cells. Hum. Immunol. 71, 541–550 (2010).
Meera, S., Madhuri, T., Manisha, G. & Ramesh, P. Irreversible loss of pDCs by apoptosis during early HIV infection may be a critical determinant of immune dysfunction. Viral Immunol. 23, 241–249 (2010).
Dillon, S. M. et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 48, 1–12 (2008).
Lehmann, C. et al. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS ONE 5, e11110 (2010).
Nascimbeni, M. et al. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-α expression. Blood 113, 6112–6119 (2009).
Finke, J. S., Shodell, M., Shah, K., Siegal, F. P. & Steinman, R. M. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 24, 647–652 (2004).
Killian, M. S., Fujimura, S. H., Hecht, F. M. & Levy, J. A. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 20, 1247–1252 (2006).
Martinson, J. A. et al. Dendritic cells from HIV-1 infected individuals are less responsive to Toll-like receptor (TLR) ligands. Cell. Immunol. 250, 75–84 (2007).
Said, E. A. et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nature Med. 16, 452–459 (2010).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 12, 1365–1371 (2006).
Kamga, I. et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J. Infect. Dis. 192, 303–310 (2005).
Sachdeva, N., Asthana, V., Brewer, T. H., Garcia, D. & Asthana, D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-α but not proinflammatory cytokines. J. Immunol. 181, 2887–2897 (2008).
Lehmann, C. et al. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 48, 522–530 (2008).
Manches, O. et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118, 3431–3439 (2008). This paper showed that HIV-stimulated pDCs induce T Reg cell differentiation through upregulation of IDO.
Favre, D. et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2, 32ra36 (2010).
Herbeuval, J. P. et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl Acad. Sci. USA 102, 13974–13979 (2005).
Herbeuval, J. P. et al. Differential expression of IFN-α and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc. Natl Acad. Sci. USA 103, 7000–7005 (2006).
Meier, A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature Med. 15, 955–959 (2009).
McMichael, A. J. HIV vaccines. Annu. Rev. Immunol. 24, 227–255 (2006).
Manches, O. & Bhardwaj, N. Resolution of immune activation defines nonpathogenic SIV infection. J. Clin. Invest. 119, 3512–3515 (2009).
Mandl, J. N. et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nature Med. 14, 1077–1087 (2008).
Bosinger, S. E. et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119, 3556–3572 (2009).
Jacquelin, B. et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119, 3544–3555 (2009).
Li, Q. et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458, 1034–1038 (2009).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Fernandez, N. C. et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nature Med. 5, 405–411 (1999).
Mailliard, R. B. et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171, 2366–2373 (2003).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Lanier, L. L. Up on the tightrope: natural killer cell activation and inhibition. Nature Immunol. 9, 495–502 (2008).
Romagnani, C. et al. CD56brightCD16− killer Ig-like receptor− NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 178, 4947–4955 (2007).
Yu, J. et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 115, 274–281 (2010).
Mavilio, D. et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl Acad. Sci. USA 102, 2886–2891 (2005).
Alter, G. et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106, 3366–3369 (2005).
Gonzalez, V. D. et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-α and ribavirin treatment. J. Immunol. 183, 6612–6618 (2009).
Lu, X. et al. CD16+ CD56− NK cells in the peripheral blood of cord blood transplant recipients: a unique subset of NK cells possibly associated with graft-versus-leukemia effect. Eur. J. Haematol. 81, 18–25 (2008).
Stratov, I., Chung, A. & Kent, S. J. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J. Virol. 82, 5450–5459 (2008).
Tiemessen, C. T. et al. Cutting edge: unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J. Immunol. 182, 5914–5918 (2009).
Collins, K. L., Chen, B. K., Kalams, S. A., Walker, B. D. & Baltimore, D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391, 397–401 (1998).
Cohen, G. B. et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10, 661–671 (1999).
Bonaparte, M. I. & Barker, E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104, 2087–2094 (2004).
Cerboni, C. et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen. Virol. 88, 242–250 (2007).
Kottilil, S. et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187, 1038–1045 (2003).
Portales, P. et al. Interferon-α restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS 17, 495–504 (2003).
De Maria, A. et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol. 33, 2410–2418 (2003).
Vieillard, V., Strominger, J. L. & Debre, P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl Acad. Sci. USA 102, 10981–10986 (2005).
Fogli, M. et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur. J. Immunol. 34, 2313–2321 (2004).
Mela, C. M. et al. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS 19, 1761–1769 (2005).
Guma, M. et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104, 3664–3671 (2004).
Bernstein, H. B. et al. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology 387, 59–66 (2009).
Flores-Villanueva, P. O. et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl Acad. Sci. USA 98, 5140–5145 (2001).
Martin, M. P. et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature Genet. 31, 429–434 (2002). This study first demonstrated the protective effect of KIR–HLA combinations in HIV-1 disease progression and showed that individuals expressing KIR3DS1 in conjunction with its putative ligand (HLA-Bw4-80I) have a slower progression to AIDS compared with individuals lacking either or both receptor–ligand pairs.
Alter, G. et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J. Virol. 83, 6798–6805 (2009).
Alter, G. et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204, 3027–3036 (2007).
Long, B. R. et al. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J. Virol. 82, 4785–4792 (2008).
Boulet, S. et al. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22, 595–599 (2008).
Gillespie, G. M. et al. Lack of KIR3DS1 binding to MHC class I Bw4 tetramers in complex with CD8+ T cell epitopes. AIDS Res. Hum. Retroviruses 23, 451–455 (2007).
Carr, W. H. et al. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J. Immunol. 178, 647–651 (2007).
Altfeld, M. & Goulder, P. 'Unleashed' natural killers hinder HIV. Nature Genet. 39, 708–710 (2007).
Hickman, H. D. et al. Cutting edge: class I presentation of host peptides following HIV infection. J. Immunol. 171, 22–26 (2003).
Lee, S. H. et al. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nature Genet. 28, 42–45 (2001).
Kielczewska, A. et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 206, 515–523 (2009).
Martin, M. P. et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nature Genet. 39, 733–740 (2007). This large genetic study showed that individuals with a KIR3DL1hiHLA-Bw4-80I+ phenotype had slower HIV-1 disease progression than individuals with a KIR3DL1lowHLA-Bw4-80I+ phenotype, indicating that KIR–HLA interactions can enhance the protective effect of HLA-Bw4 alleles.
Yawata, M. et al. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J. Exp. Med. 203, 633–645 (2006).
Thomas, R. et al. Novel KIR3DL1 alleles and their expression levels on NK cells: convergent evolution of KIR3DL1 phenotype variation? J. Immunol. 180, 6743–6750 (2008).
Kim, S. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005).
Fernandez, N. C. et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105, 4416–4423 (2005).
Anfossi, N. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006).
Kim, S. et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl Acad. Sci. USA 105, 3053–3058 (2008).
Fellay, J. et al. A whole-genome association study of major determinants for host control of HIV-1. Science 317, 944–947 (2007). This genome-wide association study showed that a protective SNP associated with higher HLA-C expression correlates with better control of HIV-1 viraemia.
Thomas, R. et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nature Genet. 41, 1290–1294 (2009).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
O'Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nature Immunol. 7, 507–516 (2006).
Kikuchi, T. et al. Dendritic cells pulsed with live and dead Legionella pneumophila elicit distinct immune responses. J. Immunol. 172, 1727–1734 (2004).
Gerosa, F. et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195, 327–333 (2002).
Gerosa, F. et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 174, 727–734 (2005).
Borg, C. et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104, 3267–3275 (2004).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nature Immunol. 5, 1260–1265 (2004).
Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N. M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Della Chiesa, M. et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur. J. Immunol. 33, 1657–1666 (2003).
Reitano, K. N. et al. Defective plasmacytoid dendritic cell–NK cell cross-talk in HIV infection. AIDS Res. Hum. Retroviruses 25, 1029–1037 (2009).
Tasca, S. et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS 17, 2291–2298 (2003).
Mavilio, D. et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 203, 2339–2350 (2006). This paper shows that NK cell-mediated DC editing is severely impaired in progressive HIV-1 infection, and this appears to be due to an increased accumulation of CD56− NK cells with impaired NKp30 function.
Quaranta, M. G. et al. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56dim and CD56bright NK cell subsets. FASEB J. 21, 2323–2334 (2007).
Poggi, A. et al. NK cell activation by dendritic cells is dependent on LFA-1-mediated induction of calcium-calmodulin kinase II: inhibition by HIV-1 Tat C-terminal domain. J. Immunol. 168, 95–101 (2002).
Melki, M. T., Saidi, H., Dufour, A., Olivo-Marin, J. C. & Gougeon, M. L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk—a pivotal role of HMGB1. PLoS Pathog. 6, e1000862 (2010).
Saidi, H., Melki, M. T. & Gougeon, M. L. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS ONE 3, e3601 (2008).
Alter, G. et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J. Clin. Invest. 120, 1905–1913 (2010). This work showed that IL-10 secreted in response to HIV-1 renders immature DCs resistant to NK cell-mediated killing, resulting in the accumulation of poorly immunogenic DCs in the lymph nodes of infected patients.
Lu, W., Arraes, L. C., Ferreira, W. T. & Andrieu, J. M. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nature Med. 10, 1359–1365 (2004).
Hansen, S. G. et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature Med. 15, 293–299 (2009).
Wyand, M. S. et al. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73, 8356–8363 (1999).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature Immunol. 11, 1127–1135 (2010).
Brodin, P., Lakshmikanth, T., Johansson, S., Karre, K. & Hoglund, P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113, 2434–2441 (2009).
Yu, J. et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J. Immunol. 179, 5977–5989 (2007).
Yawata, M. et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 112, 2369–2380 (2008).
Acknowledgements
This work was supported by grants from the US National Institutes of Health (to N.B. and M.A.), the Doris Duke Charitable Foundation (to M.A.), the Bill & Melinda Gates Foundation (to N.B. and M.A.) and the Phillip T. and Susan M. Ragon Foundation (to M.A. and L.F.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Nina Bhardwaj is an inventor on patents related to the use of dendritic cells as immunomodulators.
Glossary
- Viral synapse
-
A polarized synaptic contact point for cell to cell transmission of viruses. These contacts can occur between DCs and CD4+ T cells.
- Clusterin
-
A ubiquitously expressed glycoprotein that functions as an extracellular chaperone. It is widely expressed in various types of cancer.
- Birbeck granule
-
A membrane-bound structure that is found in the cytoplasm of Langerhans cells. These granules are rod- or tennis racket-shaped with a central linear density. Their formation is induced by langerin, an endocytic C-type lectin receptor that is specific to Langerhans cells.
- Autophagy
-
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes.
- Perforin
-
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter target cells.
- Granzyme A
-
A member of a family of serine proteinases that are found, primarily, in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. Granzymes enter target cells through perforin pores, then cleave and activate intracellular caspases to initiate target cell apoptosis.
Rights and permissions
About this article
Cite this article
Altfeld, M., Fadda, L., Frleta, D. et al. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 11, 176–186 (2011). https://doi.org/10.1038/nri2935
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2935
This article is cited by
-
Direct antiviral agents upregulate natural killer cell potential activity in chronic hepatitis C patients
Clinical and Experimental Medicine (2019)
-
Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells
AIDS Research and Therapy (2018)
-
Adenosine signaling and adenosine deaminase regulation of immune responses: impact on the immunopathogenesis of HIV infection
Purinergic Signalling (2018)
-
HIV and decreased risk of multiple sclerosis: role of low CD4+ lymphocyte count and male prevalence
Journal of NeuroVirology (2017)
-
Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV
Protein & Cell (2017)