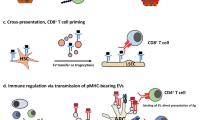
Key Points
-
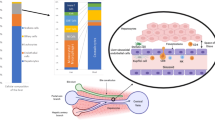
The liver is a solid organ with unique immunoregulatory functions that are determined by the hepatic microenvironment, which is rich in regulatory soluble mediators, and by local antigen-presenting cells (APCs) with tolerogenic capabilities located within a unique anatomical microarchitecture.
-
Local hepatic APCs with tolerogenic function are myeloid and plasmacytoid dendritic cells, liver sinusoidal endothelial cells, Kupffer cells and hepatocytes. Under steady-state conditions, these cells induce T cell tolerance by numerous mechanisms, including clonal elimination, the induction of T cell anergy and the induction, recruitment or proliferation of regulatory T (TReg) cells.
-
Tolerogenic hepatic APCs characteristically resist functional maturation in response to pathogen- or danger-associated molecular patterns (PAMPs; DAMPs), which are present physiologically in portal venous blood, through the development of hyporesponsiveness towards these stimuli or through non-responsiveness due to cell-intrinsic regulatory mechanisms.
-
Microbial infection leading to the functional maturation of tolerogenic into immunogenic APCs, either by cell-autonomous mechanisms or cell–cell interactions, can result in the local induction of T cell immunity in the liver by mechanisms that still need to be defined.
-
The abundance of tolerogenic APCs within the hepatic sinusoids facilitates interaction with circulating T cells and allows the liver to function as a large immunoregulatory platform aimed at skewing hepatic, as well as extrahepatic, immune responses. The principles governing hepatic tolerance or immunity may be exploited to develop therapeutic options to mitigate autoimmunity or allograft rejection, to prolong hepatic transgene expression or to overcome tolerogenic barriers in persistent infection and cancer.
Abstract
The demands that are imposed on the liver as a result of its function as a metabolic organ that extracts nutrients and clears gut-derived microbial products from the blood are met by a unique microanatomical and immunological environment. The inherent tolerogenicity of the liver and its role in the regulation of innate and adaptive immunity are mediated by parenchymal and non-parenchymal antigen-presenting cells (APCs), cell-autonomous molecular pathways and locally produced factors. Here, we review the central role of liver APCs in the regulation of hepatic immune function and also consider how recent insights may be applied in strategies to target liver tolerance for disease therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Breiner, K. M., Schaller, H. & Knolle, P. A. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology 34, 803–808 (2001).
Cormier, E. G. et al. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc. Natl Acad. Sci. USA 101, 14067–14072 (2004).
Pradel, G. & Frevert, U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 33, 1154–1165 (2001).
van Egmond, M. et al. FcαRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nature Med. 6, 680–685 (2000).
Helmy, K. Y. et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006).
Inatsu, A. et al. Novel mechanism of C-reactive protein for enhancing mouse liver innate immunity. Hepatology 49, 2044–2054 (2009).
Knolle, P. A. & Gerken, G. Local control of the immune response in the liver. Immunol. Rev. 174, 21–34 (2000).
Das, A. et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205, 2111–2124 (2008).
Ball, H. J., Yuasa, H. J., Austin, C. J., Weiser, S. & Hunt, N. H. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int. J. Biochem. Cell Biol. 41, 467–471 (2009).
Steptoe, R. J., Patel, R. K., Subbotin, V. M. & Thomson, A. W. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: morphometric estimation in normal mice. Transpl. Immunol. 8, 49–56 (2000).
Woo, J. et al. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation 58, 484–491 (1994).
Steptoe, R. J. et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J. Immunol. 159, 5483–5491 (1997).
Lu, L. et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J. Exp. Med. 179, 1823–1834 (1994). Together with references 14 and 64, these findings provide insight into the regulation of liver DC maturation and are congruent with the possibility that the migration of immature DCs from liver grafts may explain their inherent tolerogenicity.
Rastellini, C. et al. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 60, 1366–1370 (1995).
Thomson, A. W. & Lu, L. Are dendritic cells the key to liver transplant tolerance? Immunol. Today 20, 27–32 (1999).
Bamboat, Z. M. et al. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 182, 1901–1911 (2009). These observations show that human liver DCs secrete substantial amounts of IL-10, induce T cell hyporesponsiveness and generate T Reg cells by an IL-10-dependent mechanism.
Xia, S. et al. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 112, 3175–3185 (2008). This study provides evidence that the liver microenvironment is crucial for programming haematopoietic progenitor cells to develop into tolerogenic DCs in situ , a process that may contribute to the maintenance of hepatic tolerance.
Li, G., Kim, Y. J. & Broxmeyer, H. E. Macrophage colony-stimulating factor drives cord blood monocyte differentiation into IL-10highIL-12absent dendritic cells with tolerogenic potential. J. Immunol. 174, 4706–4717 (2005).
Rutella, S. et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 108, 218–227 (2006).
Xia, G., He, J. & Leventhal, J. R. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am. J. Transplant. 8, 298–306 (2008).
Cabillic, F. et al. Hepatic environment elicits monocyte differentiation into a dendritic cell subset directing TH2 response. J. Hepatology 44, 552–559 (2006).
Lian, Z. X. et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J. Immunol. 170, 2323–2330 (2003).
Pillarisetty, V. G., Shah, A. B., Miller, G., Bleier, J. I. & DeMatteo, R. P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 172, 1009–1017 (2004).
O'Connell, P. J., Morelli, A. E., Logar, A. J. & Thomson, A. W. Phenotypic and functional characterization of mouse hepatic CD8α+ lymphoid-related dendritic cells. J. Immunol. 165, 795–803 (2000).
Swiecki, M. & Colonna, M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234, 142–162 (2010).
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007).
Naik, S. H. et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nature Immunol. 8, 1217–1226 (2007).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Bosma, B. et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 12, 384–393 (2006).
Tanis, W. et al. Human hepatic lymph nodes contain normal numbers of mature myeloid dendritic cells but few plasmacytoid dendritic cells. Clin. Immunol. 110, 81–88 (2004).
Yoneyama, H. et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193, 35–50 (2000).
Matsuno, K., Nomiyama, H., Yoneyama, H. & Uwatoku, R. Kupffer cell-mediated recruitment of dendritic cells to the liver crucial for a host defense. Dev. Immunol. 9, 143–149 (2002).
Uwatoku, R. et al. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology 121, 1460–1472 (2001).
Matsuno, K., Ezaki, T., Kudo, S. & Uehara, Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis and translocation from the liver to the draining lymph. J. Exp. Med. 183, 1865–1878 (1996).
Sato, T., Yamamoto, H., Sasaki, C. & Wake, K. Maturation of rat dendritic cells during intrahepatic translocation evaluated using monoclonal antibodies and electron microscopy. Cell Tissue Res. 294, 503–514 (1998).
Kudo, S., Matsuno, K., Ezaki, T. & Ogawa, M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J. Exp. Med. 185, 777–784 (1997).
Yrlid, U. et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Abe, M., Zahorchak, A. F., Colvin, B. L. & Thomson, A. W. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation 78, 762–765 (2004).
van den Oord, J. J. et al. Distribution of non-lymphoid, inflammatory cells in chronic HBV infection. J. Pathol. 160, 223–230 (1990).
Yoneyama, H. et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193, 35–49 (2001).
Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Abe, M., Tokita, D., Raimondi, G. & Thomson, A. W. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur. J. Immunol. 36, 2483–2493 (2006).
De Creus, A. et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 174, 2037–2045 (2005).
Chen, Y. et al. Distinct response of liver myeloid dendritic cells to endotoxin is mediated by IL-27. J. Hepatol. 51, 510–519 (2009).
Khanna, A. et al. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J. Immunol. 164, 1346–1354 (2000).
Jinushi, M. et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4+ CD25+ T cells with PD-1-dependent regulatory activities. Immunology 120, 73–82 (2007).
Kingham, T. P. et al. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology 45, 445–454 (2007).
Villadangos, J. A. & Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008).
Castellaneta, A., Sumpter, T. L., Chen, L., Tokita, D. & Thomson, A. W. NOD2 ligation subverts IFN-α production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J. Immunol. 183, 6922–6932 (2009).
Jomantaite, I. et al. Hepatic dendritic cell subsets in the mouse. Eur. J. Immunol. 34, 355–365 (2004).
Fritz, J. H., Ferrero, R. L., Philpott, D. J. & Girardin, S. E. Nod-like proteins in immunity, inflammation and disease. Nature Immunol. 7, 1250–1257 (2006).
Tokita, D. et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J. Hepatol. 49, 1008–1018 (2008).
Goubier, A. et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475 (2008). Evidence that liver pDCs induce efficient CD4+ and CD8+ T cell tolerance to orally administered antigens that reach the liver through the blood.
Watanabe, T. et al. A liver tolerates a portal antigen by generating CD11c+ cells, which select Fas ligand+ TH2 cells via apoptosis. Hepatology 38, 403–412 (2003).
Crispe, I. N. Hepatic T cells and liver tolerance. Nature Rev. Immunol. 3, 51–62 (2003). A detailed account of T cell biology in the liver and of the mechanisms that promote T cell inactivation, tolerance and apoptosis following local antigen presentation in the liver.
Tsoulfas, G. et al. Activation of the lipopolysaccharide signaling pathway in hepatic transplantion preservation injury. Transplantation 74, 7–13 (2002).
Zhai, Y. et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 173, 7115–7119 (2004).
Tsung, A. et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J. Immunol. 175, 7661–7668 (2005).
Loi, P. et al. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant. Proc. 36, 1275–1279 (2004).
Bamboat, Z. M. et al. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J. Clin. Invest. 120, 559–569 (2010).
Tsung, A. et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J. Leuk. Biol. 81, 1–10 (2007).
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 8, 487–496 (2007).
Calne, R. Y. et al. Induction of immunological tolerance by porcine liver allografts. Nature 223, 472–476 (1969).
Lu, L. et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony stimulating factor. J. Exp. Med. 182, 379–387 (1995).
Drakes, M. L., Lu, L., Subbotin, V. M. & Thomson, A. W. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J. Immunol. 159, 4268–4278 (1997).
Morelli, A. E. et al. Preferential induction of TH1 responses by functionally mature hepatic (CD8α− and CD8α+) dendritic cells: association with conversion from liver transplant tolerance to acute rejection. Transplantation 69, 2647–2657 (2000).
Li, W. et al. IL-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J. Immunol. 166, 5619–5628 (2001).
Wysocka, M., Montaner, L. J. & Karp, C. L. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. J. Immunol. 174, 7398–7402 (2005).
Chen, S. et al. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 148, 49–57 (2000).
Zhang, Z. et al. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin. Immunol. 122, 173–180 (2007).
Lai, W. K. et al. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J. Hepatol. 47, 338–347 (2007).
Lau, D. T. et al. Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology 47, 799–809 (2008).
Takahashi, K. et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc. Natl Acad. Sci. USA 107, 7431–7436 (2010).
Kinoshita, M. et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 53, 903–910 (2010).
Hardonk, M. J., Dijkhuis, F. W., Grond, J., Koudstaal, J. & Poppema, S. Evidence for a migratory capability of rat Kupffer cells to portal tracts and hepatic lymph nodes. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 51, 429–442 (1986).
Ju, C., McCoy, J. P., Chung, C. J., Graf, M. L. & Pohl, L. R. Tolerogenic role of Kupffer cells in allergic reactions. Chem. Res. Toxicol. 16, 1514–1519 (2003).
Callery, M. P., Kamei, T. & Flye, M. W. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation 47, 1092–1094 (1989).
Sato, K., Yabuki, K., Haba, T. & Maekawa, T. Role of Kupffer cells in the induction of tolerance after liver transplantation. J. Surg. Res. 63, 433–438 (1996).
You, Q., Cheng, L., Kedl, R. M. & Ju, C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 48, 978–990 (2008).
Knolle, P. A. et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology 27, 93–99 (1998).
Bissell, D. M., Wang, S. S., Jarnagin, W. R. & Roll, F. J. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J. Clin. Invest. 96, 447–455 (1995).
Breous, E., Somanathan, S., Vandenberghe, L. H. & Wilson, J. M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50, 612–621 (2009). This report and references 79 and 80 describe mechanisms whereby Kupffer cells support systemic T cell tolerance towards circulating and hepatocyte-derived antigens.
Wiegard, C. et al. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology 42, 193–199 (2005).
Kuniyasu, Y., Marfani, S. M., Inayat, I. B., Sheikh, S. Z. & Mehal, W. Z. Kupffer cells required for high affinity peptide-induced deletion, not retention, of activated CD8+ T cells by mouse liver. Hepatology 39, 1017–1027 (2004). Evidence that Kupffer cells are required for the deletion, not retention, of activated CD8+ T cells in the liver.
Polakos, N. K. et al. Kupffer cell-dependent hepatitis occurs during influenza infection. Am. J. Pathol. 168, 1169–1178 (2006).
Montalvo-Jave, E. E., Escalante-Tattersfield, T., Ortega-Salgado, J. A., Pina, E. & Geller, D. A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 147, 153–159 (2008).
Giakoustidis, D. E. et al. Blockade of Kupffer cells by gadolinium chloride reduces lipid peroxidation and protects liver from ischemia/reperfusion injury. Hepatogastroenterology 50, 1587–1592 (2003).
Ellett, J. D. et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J. Immunol. 184, 5849–5858 (2010).
Schmieg, J., Yang, G., Franck, R. W., Van Rooijen, N. & Tsuji, M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc. Natl Acad. Sci. USA 102, 1127–1132 (2005).
Beattie, L. et al. Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog. 6, e1000805 (2010).
Lee, W. Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nature Immunol. 11, 295–302 (2010). This article reports a major advance in imaging technology for visualizing the interaction of hepatic APCs with pathogens. The new technology has revealed a novel role for Kupffer cells in cooperation with hepatic NKT cells in antibacterial immunity.
Giannandrea, M., Pierce, R. H. & Crispe, I. N. Indirect action of tumor necrosis factor-α in liver injury during the CD8+ T cell response to an adeno-associated virus vector in mice. Hepatology 49, 2010–2020 (2009).
Knolle, P. A. et al. Induction of cytokine production in naive CD4+ T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward TH1 cells. Gastroenterology 116, 1428–1440 (1999). This report provides early evidence and the basis for further work showing that LSECs present antigen and promote T cell tolerance.
Lohse, A. W. et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 110, 1175–1181 (1996).
Knolle, P. A. et al. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J. Immunol. 162, 1401–1407 (1999).
Onoe, T. et al. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. J. Immunol. 175, 139–146 (2005).
Tokita, D. et al. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J. Immunol. 177, 3615–3624 (2006).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Med. 6, 1348–1354 (2000). This report indicates that the outcome of soluble exogenous antigen cross-presentation by LSECs to CD8+ T cells is tolerance rather than immunity.
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Schurich, A. et al. Distinct kinetics and dynamics of cross-presentation in liver sinusoidal endothelial cells compared to dendritic cells. Hepatology 50, 909–919 (2009).
Diehl, L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47, 296–305 (2008).
Limmer, A. et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 35, 2970–2981 (2005).
Berg, M. et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur. J. Immunol. 36, 2960–2970 (2006).
Schurich, A. et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J. Immunol. 184, 4107–4114 (2010).
Pohlmann, S. et al. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77, 4070–4080 (2003).
Kern, M. et al. Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity. Gastroenterology 138, 336–346 (2010).
Jacob, A. I., Goldberg, P. K., Bloom, N., Degenshein, G. A. & Kozinn, P. J. Endotoxin and bacteria in portal blood. Gastroenterology 72, 1268–1270 (1977).
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R. M. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
Bashirova, A. A. et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193, 671–678 (2001).
Liu, W. et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 279, 18748–18758 (2004).
Geijtenbeek, T. B. & Gringhuis, S. I. Signalling through C-type lectin receptors: shaping immune responses. Nature Rev. Immunol. 9, 465–479 (2009).
Tang, L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508 (2009). These findings reveal that LSECtin, which is expressed by LSECs, is a new negative regulator of hepatic T cell function.
Dong, H. et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 20, 327–336 (2004). Evidence that the co-inhibitory molecule B7-H1 selectively regulates the accumulation and deletion of CD8+ T cells in the liver.
Schildberg, F. A. et al. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur. J. Immunol. 38, 957–967 (2008).
Warren, A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44, 1182–1190 (2006).
Bertolino, P., Trescol-Biemont, M. C. & Rabourdin-Combe, C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 28, 221–236 (1998).
Holz, L. E. et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology 135, 989–997 (2008).
Bowen, D. G. et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 114, 701–712 (2004). References 116–118 provide evidence that intrahepatic T cell activation by hepatocytes leads to (BIM-dependent) death and promotes intrahepatic tolerance.
Wahl, C., Bochtler, P., Schirmbeck, R. & Reimann, J. Type I IFN-producing CD4 Vα14 iNKT cells facilitate priming of IL-10-producing CD8 T cells by hepatocytes. J. Immunol. 178, 2083–2093 (2007).
Herkel, J. et al. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocytes. Hepatology 37, 1079–1085 (2003).
Wiegard, C. et al. Defective T helper response of hepatocyte-stimulated CD4 T cells impairs antiviral CD8 response and viral clearance. Gastroenterology 133, 2010–2018 (2007).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Dikopoulos, N. et al. Recently primed CD8+ T cells entering the liver induce hepatocytes to interact with naive CD8+ T cells in the mouse. Hepatology 39, 1256–1266 (2004).
Wuensch, S. A., Pierce, R. H. & Crispe, I. N. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J. Immunol. 177, 1689–1697 (2006).
Vinas, O. et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 38, 919–929 (2003).
Winau, F. et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26, 117–129 (2007).
Yu, M. C. et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321 (2004).
Yang, H. R. et al. A critical role of TRAIL expressed on cotransplanted hepatic stellate cells in prevention of islet allograft rejection. Microsurgery 30, 332–337 (2010).
Jiang, G. et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation 86, 1492–1502 (2008).
Yang, H. R. et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-γ signaling. Hepatology 50, 1981–1991 (2009).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature Med. 12, 342–347 (2006).
Mingozzi, F. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nature Med. 13, 419–422 (2007).
Pien, G. C. et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 119, 1688–1695 (2009).
Cooper, M. et al. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 20, 767–776 (2009).
Mingozzi, F. et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 110, 2334–2341 (2007).
Kren, B. T. et al. Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice. J. Clin. Invest. 119, 2086–2099 (2009).
Feng, S. Long-term management of immunosuppression after pediatric liver transplantation: is minimization or withdrawal desirable or possible or both? Curr. Opin. Organ Transplant. 13, 506–512 (2008).
Orlando, G., Soker, S. & Wood, K. Operational tolerance after liver transplantation. J. Hepatol. 50, 1247–1257 (2009).
Horstmann, B., Zinser, E., Turza, N., Kerek, F. & Steinkasserer, A. MCS-18, a novel natural product isolated from Helleborus purpurascens, inhibits dendritic cell activation and prevents autoimmunity as shown in vivo using the EAE model. Immunobiology 212, 839–853 (2007).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Luth, S. et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J. Clin. Invest. 118, 3403–3410 (2008). An encouraging report showing that ectopic expression of an autoantigen in the liver can promote liver-induced tolerance and suppress extrahepatic autoimmune disease.
Junt, T., Scandella, E. & Ludewig, B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nature Rev. Immunol. 8, 764–775 (2008).
MacPhee, P. J., Schmidt, E. E. & Groom, A. C. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am. J. Physiol. 269, G692–G698 (1995).
Wong, J. et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest. 99, 2782–2790 (1997).
Bertolino, P., Bowen, D. G., McCaughan, G. W. & Fazekas de St. Groth, B. Antigen-specific primary activation of CD8+ T cells within the liver. J. Immunol. 166, 5430–5438 (2001).
von Oppen, N. et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 49, 1664–1672 (2009).
Uwatoku, R. et al. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology 121, 1460–1472 (2001).
Schrage, A. et al. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology 48, 1262–1272 (2008).
Oo, Y. H. & Adams, D. H. The role of chemokines in the recruitment of lymphocytes to the liver. J. Autoimmun. 34, 45–54 (2010).
Bonder, C. S. et al. Rules of recruitment for TH1 and TH2 lymphocytes in inflamed liver: a role for α4 integrin and vascular adhesion protein-1. Immunity 23, 153–163 (2005).
John, B. & Crispe, I. N. Passive and active mechanisms trap activated CD8+ T cells in the liver. J. Immunol. 172, 5222–5229 (2004).
Oo, Y. H. et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J. Immunol. 184, 2886–2898 (2010).
Adams, D. H. & Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nature Rev. Immunol. 6, 244–251 (2006).
Kruse, N. et al. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3− regulatory T cells suppressing autoimmune hepatitis. Hepatology 50, 1904–1913 (2009).
Huehn, J. & Hamann, A. Homing to suppress: address codes for Treg migration. Trends Immunol. 26, 632–636 (2005).
Braet, F. & Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 1, 1 (2002).
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Adams, D. H., Eksteen, B. & Curbishley, S. M. Immunology of the gut and liver: a love/hate relationship. Gut 57, 838–848 (2008).
Lunz, J. G., Specht, S. M., Murase, N., Isse, K. & Demetris, A. J. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity. Hepatology 46, 1946–1959 (2007).
Chu, C. L. et al. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRγ. Eur. J. Immunol. 38, 166–173 (2008).
Sumpter, T. L., Lunz, J. G., Demetris, A. J. & Thomson, A. W. Molecular regulation of hepatic dendritic cell function and its relation to liver transplant outcome. Transplantation 88, S40–S44 (2009).
Wahl, C., Bochtler, P., Chen, L., Schirmbeck, R. & Reimann, J. B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology 135, 980–988 (2008).
O'Connell, P. J. et al. Type-1 polarized nature of mouse liver CD8α− and CD8α+ dendritic cells: tissue-dependent differences offset CD8α-related dendritic cell heterogeneity. Eur. J. Immunol. 33, 2007–2013 (2003).
Dubois, B. et al. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology 137, 1019–1028 (2009).
Bertolino, P., Heath, W. R., Hardy, C. L., Morahan, G. & Miller, J. F. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur. J. Immunol. 25, 1932–1942 (1995).
Acknowledgements
The authors' work is currently supported by US National Institutes of Health grant P01 AI81678 and the Roche Organ Transplantation Research Foundation (874,279,717) (to A.W.T.) and by DFG grants SFB 704, SFB TR57, GRK 804 and SFB 670 (to P.A.K.).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Oral tolerance
-
The immunological mechanism whereby the mucosal immune system maintains unresponsiveness to antigens in the mucosal environment that might otherwise induce undesired immune responses.
- Portal venous tolerance
-
The induction of peripheral tolerance following portal venous delivery of antigen (most commonly alloantigen).
- Reticulo-endothelial system
-
The general phagocytic system of the host. It is responsible for the removal and destruction of foreign material and senescent or dead host cells, such as red blood cells.
- Transcytosis
-
The process of transport of material across a cell layer by uptake on one side of the cell into a coated vesicle. The vesicle might then be sorted through the trans-Golgi network and transported to the opposite side of the cell.
- Pattern recognition receptor
-
(PRR). A host receptor (such as Toll-like receptors (TLRs) or NOD-like receptors (NLRs)) that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immunity. These receptors can be membrane bound (such as TLRs) or soluble cytoplasmic receptors (such as retinoic acid-inducible gene-I, melanoma differentiation associated gene 5 or NLRs).
- Glycocalyx
-
A carbohydrate-rich coating that covers the outside of many eukaryotic and prokaryotic cells, particularly bacteria, and which, on bacterial cells, provides a protective coat from host factors.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecular patterns that are found in pathogens but not mammalian cells. Examples include terminally mannosylated and polymannosylated compounds, which bind the mannose receptor, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA, which bind to Toll-like receptors.
- Acute phase proteins
-
A group of proteins, including C-reactive protein, serum amyloid A, fibrinogen and α1-acid glycoprotein, that are secreted into the blood in increased or decreased quantities by hepatocytes in response to trauma, inflammation or disease. These proteins can be inhibitors or mediators of inflammatory processes.
- Indoleamine 2,3-dioxygenase
-
(IDO). An intracellular haem-containing enzyme that catalyses the oxidative catabolism of tryptophan. Insufficient availability of tryptophan can lead to T cell apoptosis and anergy.
- Glisson's capsule
-
The capsule of the liver. A layer of connective tissue that surrounds the liver and ensheathes the hepatic artery, portal vein and bile ducts within the liver.
- FMS-like tyrosine kinase 3 ligand
-
(FLT3L). An endogenous cytokine that stimulates the proliferation of stem and progenitor cells through binding to the FLT3 receptor (a type III receptor tyrosine kinase member of the platelet-derived growth factor family). FLT3L administration substantially increases the number of dendritic cells in lymphoid and non-lymphoid tissues.
- Portal-associated lymphoid tissue
-
(PALT). An inducible, liver-specific immune tissue that may act as a first line of defence of the lymphatic pathway, as well as a site of local induction of immunity in the liver.
- Ischaemia–reperfusion injury
-
Cellular damage caused by the return of a blood supply to a tissue after a period of inadequate blood supply. The absence of oxygen and nutrients causes cellular damage such that restoration of the blood flow results in inflammation.
- Danger-associated molecular pattern
-
(DAMP). As a result of cellular stress, cellular damage and non-physiological cell death, DAMPs are released from the degraded stroma (for example, hyaluronate), from the nucleus (for example, high-mobility group box 1 protein (HMGB1)) and from the cytoplasm (for example, adenosine triphosphate, uric acid, S100 calcium-binding proteins and heat-shock proteins). Such DAMPs are thought to elicit local inflammatory reactions.
Rights and permissions
About this article
Cite this article
Thomson, A., Knolle, P. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 10, 753–766 (2010). https://doi.org/10.1038/nri2858
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2858