Key Points
-
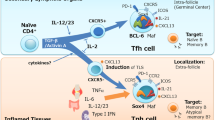
T follicular helper (TFH) cells are a specialized subset of CD4+ T helper (TH) cells that are found in the B cell follicle during germinal centre reactions. TFH cells provide help to B cells through the provision of soluble factors and cell surface molecules that promote the production of high-affinity class-switched antibodies.
-
TFH cells express several molecules in common with all recently activated CD4+ T cells but are distinguished from other fully differentiated TH cell subsets by their germinal centre location and by continued high expression of CXC-chemokine receptor 5 and interleukin-21.
-
Support for the lineage specification of TFH cells comes from recent studies showing that the transcriptional repressor B cell lymphoma 6 (BCL-6) is required for TFH cell differentiation. Studies suggest that BCL-6 suppresses the differentiation of TH1 and TH17 cells and supports the differentiation of TFH cells.
-
The differentiation pathway of TFH cells remains controversial. Recent studies have focused on the relationship between TFH cells and other TH cell subsets. Interaction with, and adhesion to, B cells is crucial for 'selecting' TFH cells from a pool of high-affinity T cell clones, and recent studies support the notion that TFH cells and other TH cell subsets share common cytokine-competent precursors and retain a degree of plasticity during their differentiation.
-
TFH cells express cell surface markers that are associated with exhausted T cells. However, the identification of TFH cells that remain in B cell follicles after the resolution of the germinal centre reaction offers insight into the fate of TFH cells.
-
TFH cells that deliver inappropriate helper signals to B cells are likely to underpin antibody-mediated autoimmune diseases, and studies indicate that certain T cell lymphomas share many similarities with those of TFH cells.
Abstract
The seminal studies characterizing T follicular helper (TFH) cells described a non-polarized CD4+ T cell population with a unique ability to home to B cell follicles and to induce antibody production by B cells. In the past few years, the study of TFH cells has enjoyed a renaissance and there has been a surge of research activity aimed at understanding the function and differentiation of these important cells. This Review focuses on the current progress in TFH cell biology and the important questions that remain unanswered. Particular attention is paid to recent studies that support the idea that TFH cells are a separate T cell lineage and those that probe the relationship of TFH cells to other T helper cell subsets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
MacLennan, I. C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Kelsoe, G. The germinal center reaction. Immunol. Today 16, 324–326 (1995).
Liu, Y. J. et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity 4, 241–250 (1996).
de Vinuesa, C. G. et al. Germinal centers without T cells. J. Exp. Med. 191, 485–494 (2000).
Fuller, K. A., Kanagawa, O. & Nahm, M. H. T cells within germinal centers are specific for the immunizing antigen. J. Immunol. 151, 4505–4512 (1993).
Gulbranson-Judge, A. & MacLennan, I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur. J. Immunol. 26, 1830–1837 (1996).
Ansel, K. M., McHeyzer-Williams, L. J., Ngo, V. N., McHeyzer-Williams, M. G. & Cyster, J. G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Allen, C. D., Okada, T., Tang, H. L. & Cyster, J. G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007). Here, evidence from intravital microscopy indicates that T cell–B cell interactions in the germinal centre are weighted towards intense B cell competition for a small number of CD4+ T cells.
MacLennan, I. C., Liu, Y. J. & Johnson, G. D. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol. Rev. 126, 143–161 (1992).
Claman, H. N., Chaperon, E. A. & Triplett, R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc. Soc. Exp. Biol. Med. 122, 1167–1171 (1966).
Miller, J. F. & Mitchell, G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J. Exp. Med. 128, 801–820 (1968).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000). References 12 and 13 are seminal papers characterizing T FH cells.
Kim, C. H. et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193, 1373–1381 (2001).
Ebert, L. M., Horn, M. P., Lang, A. B. & Moser, B. B cells alter the phenotype and function of follicular-homing CXCR5+ T cells. Eur. J. Immunol. 34, 3562–3571 (2004).
Kim, C. H. et al. Unique gene expression program of human germinal center T helper cells. Blood 104, 1952–1960 (2004).
Fazilleau, N., McHeyzer-Williams, L. J., Rosen, H. & McHeyzer-Williams, M. G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature Immunol. 10, 375–384 (2009). This study used tetramer-based detection of endogenous antigen-specific T cells to show that T FH cells have higher antigen binding affinity than other T cell populations.
Haynes, N. M. et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 (2007).
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L. & Germain, R. N. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008). This study shows that SAP is crucial for stabilizing T cell–B cell interactions but unnecessary for T cell–DC interactions. Without SAP, defective T cell–B cell conjugates resulted in insufficient help to B cells and poor generation of T FH cells.
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Chang, J. T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunol. 10, 385–393 (2009). This paper shows that after parasite infection both IL-4- and IFNγ-expressing T FH cells are present in the germinal centre, where they drive antibody class switching. Most IL-4 in the lymph node is shown to be produced by T cells in the B cell follicle and germinal centre. Although IL-4+ T FH cells comprised only 1–3% of the T cells in T cell–B cell conjugates, the interacting B cells had high expression of germinal centre B cell markers and showed evidence of affinity maturation.
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nature Immunol. 8, 753–761 (2007).
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Boyton, R. J. & Altmann, D. M. Is selection for TCR affinity a factor in cytokine polarization? Trends Immunol. 23, 526–529 (2002).
Hosken, N. A., Shibuya, K., Heath, A. W., Murphy, K. M. & O'Garra, A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-αβ-transgenic model. J. Exp. Med. 182, 1579–1584 (1995).
Deenick, E. K., Hasbold, J. & Hodgkin, P. D. Decision criteria for resolving isotype switching conflicts by B cells. Eur. J. Immunol. 35, 2949–2955 (2005).
Abbas, A. K., Urioste, S., Collins, T. L. & Boom, W. H. Heterogeneity of helper/inducer T lymphocytes. IV. Stimulation of resting and activated B cells by Th1 and Th2 clones. J. Immunol. 144, 2031–2037 (1990).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998).
Toellner, K. M. et al. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 187, 1193–1204 (1998).
Cunningham, A. F. et al. Th2 activities induced during virgin T cell priming in the absence of IL-4, IL-13, and B cells. J. Immunol. 169, 2900–2906 (2002).
Toellner, K. M., Gulbranson-Judge, A., Taylor, D. R., Sze, D. M. & MacLennan, I. C. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 183, 2303–2312 (1996).
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008). This study shows that IL-21 is important for the differentiation of T FH cells and provides evidence that T FH cells are a distinct T H cell subset.
Eddahri, F. et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood 113, 2426–2433 (2009).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 8, 967–974 (2007).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005).
Batista, F. D. & Neuberger, M. S. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8, 751–759 (1998).
Forster, R., Emrich, T., Kremmer, E. & Lipp, M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84, 830–840 (1994).
Obermeier, F. et al. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur. J. Immunol. 33, 3265–74 (2003).
Akiba, H. et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175, 2340–2348 (2005).
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunol. 9, 641–649 (2008).
Rivino, L. et al. Chemokine receptor expression identifies pre-T helper (Th)1, pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200, 725–735 (2004).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Rasheed, A. U., Rahn, H. P., Sallusto, F., Lipp, M. & Muller, G. Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 36, 1892–1903 (2006).
Bryant, V. L. et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179, 8180–8190 (2007).
Grimbacher, B. et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunol. 4, 261–268 (2003).
Aruffo, A. et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72, 291–300 (1993).
DiSanto, J. P., Bonnefoy, J. Y., Gauchat, J. F., Fischer, A. & de Saint Basile, G. CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature 361, 541–543 (1993).
Korthauer, U. et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature 361, 539–541 (1993).
Banchereau, J. et al. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12, 881–922 (1994).
Oxenius, A. et al. CD40-CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+ T cell functions. J. Exp. Med. 183, 2209–2218 (1996).
van Kooten, C. & Banchereau, J. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9, 330–337 (1997).
Tafuri, A. et al. ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001).
Lohning, M. et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 197, 181–193 (2003).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177, 4927–4932 (2006).
Walker, L. S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190, 1115–1122 (1999).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Wurster, A. L. et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon γ-producing Th1 cells. J. Exp. Med. 196, 969–977 (2002).
Pesce, J. et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Suto, A., Wurster, A. L., Reiner, S. L. & Grusby, M. J. IL-21 inhibits IFN-γ production in developing Th1 cells through the repression of eomesodermin expression. J. Immunol. 177, 3721–3727 (2006).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008). This study shows that IL-21 has an important role in T FH cell differentiation that precedes the acquisition of the B cell follicle homing programme.
Ettinger, R. et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175, 7867–7879 (2005).
Good, K. L., Bryant, V. L. & Tangye, S. G. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 177, 5236–5247 (2006).
Avery, D. T., Bryant, V. L., Ma, C. S., de Waal Malefyt, R. & Tangye, S. G. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 181, 1767–1779 (2008).
Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Schwartzberg, P. L., Mueller, K. L., Qi, H. & Cannons, J. L. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nature Rev. Immunol. 9, 39–46 (2009).
Zhou, L., Chong, M. M. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Kim, J. I., Ho, I. C., Grusby, M. J. & Glimcher, L. H. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity 10, 745–751 (1999).
Pai, S. Y., Truitt, M. L. & Ho, I. C. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl Acad. Sci. USA 101, 1993–1998 (2004).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003).
Bauquet, A. T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature Immunol. 10, 167–175 (2009).
Pot, C. et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 (2009).
Fukuda, T. et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997).
Yu, D. et al. The transcriptional repressor BCL-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009). This study shows that BCL-6 repressed numerous microRNAs, which accounted for the characteristic T FH cell expression signature.
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009). Through studying the effects of overexpression of BCL-6 and its genetic deficiency, this transcriptional repressor is shown to be both sufficient and necessary for T FH cell differentiation.
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009). This paper shows that BCL-6 is the key transcription factor that controls the expression of T FH cell-associated genes and T FH cell generation.
Ree, H. J. et al. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum. Pathol. 30, 403–411 (1999).
Kusam, S., Toney, L. M., Sato, H. & Dent, A. L. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J. Immunol. 170, 2435–2441 (2003).
Allen, C. D., Okada, T. & Cyster, J. G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
Schwickert, T. A. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007).
Marinova, E., Han, S. & Zheng, B. Human germinal center T cells are unique Th cells with high propensity for apoptosis induction. Int. Immunol. 18, 1337–1345 (2006).
Zinkernagel, R. M. et al. On immunological memory. Annu. Rev. Immunol. 14, 333–367 (1996).
MacLennan, I. C. et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol. Rev. 156, 53–66 (1997).
Zaph, C., Uzonna, J., Beverley, S. M. & Scott, P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature Med. 10, 1104–1110 (2004).
King, I. L. & Mohrs, M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 206, 1001–1007 (2009).
Vieira, P. & Rajewsky, K. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 2, 487–494 (1990).
Hebeis, B. J. et al. Activation of virus-specific memory B cells in the absence of T cell help. J. Exp. Med. 199, 593–602 (2004).
Kopf, M., Le Gros, G., Coyle, A. J., Kosco-Vilbois, M. & Brombacher, F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol. Rev. 148, 45–69 (1995).
Frohlich, A. et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood 109, 2023–2031 (2007).
King, C., Tangye, S. G. & Mackay, C. R. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26, 741–766 (2008).
Cimmino, L. et al. Blimp-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J. Immunol. 181, 2338–2347 (2008).
Zaretsky, A. G. et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206, 991–999 (2009).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunol. 9, 166–175 (2008).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009). Tracking of FOXP3EGFP cells indicates that T FH cells in the Peyer's patches are preferentially generated from T Reg cell precursors, whereas those in the spleen and lymph nodes are not. T Reg cells that became T FH cells downregulate FOXP3 expression, suggesting a degree of T H cell plasticity.
Lim, H. W., Hillsamer, P. & Kim, C. H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J. Clin. Invest. 114, 1640–1649 (2004).
Lim, H. W., Hillsamer, P., Banham, A. H. & Kim, C. H. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 175, 4180–4183 (2005).
Hutloff, A. et al. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 50, 3211–3220 (2004).
Watanabe, T. et al. Striking alteration of some populations of T/B cells in systemic lupus erythematosus: relationship to expression of CD62L or some chemokine receptors. Lupus 17, 26–33 (2008).
Luzina, I. G. et al. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70, 578–584 (2001).
Herber, D. et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178, 3822–30 (2007).
Young, D. A. et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 56, 1152–1163 (2007).
Iwai, H. et al. Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. J. Immunol. 171, 2848–2854 (2003).
Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009).
Hu, Y. L., Metz, D. P., Chung, J., Siu, G. & Zhang, M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J. Immunol. 182, 1421–1428 (2009).
Grammer, A. C. et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J. Clin. Invest. 112, 1506–1520 (2003).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Vinuesa, C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005). This study shows that a single recessive mutation in roquin (also known as Rc3h1 , which encodes a RING-type ubiquitin ligase) disrupts the regulation of ICOS to expand T FH cells and germinal centres.
Rodriguez-Justo, M. et al. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres: a neoplasia with origin in the outer zone of the germinal centre? Clinicopathological and immunohistochemical study of 10 cases with follicular T-cell markers. Mod. Pathol. 22, 753–761 (2009).
de Leval, L., Savilo, E., Longtine, J., Ferry, J. A. & Harris, N. L. Peripheral T-cell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am. J. Surg. Pathol. 25, 395–400 (2001).
de Leval, L., Bisig, B., Thielen, C., Boniver, J. & Gaulard, P. Molecular classification of T-cell lymphomas. Crit. Rev. Oncol. Hematol. 23 Feb 2009 (doi:10.1016/j.critrevonc.2009.01.002).
Krenacs, L., Schaerli, P., Kis, G. & Bagdi, E. Phenotype of neoplastic cells in angioimmunoblastic T-cell lymphoma is consistent with activated follicular B helper T cells. Blood 108, 1110–1111 (2006).
Yu, H., Shahsafaei, A. & Dorfman, D. M. Germinal-center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma. Am. J. Clin. Pathol. 131, 33–41 (2009).
Grogg, K. L. et al. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod. Pathol. 19, 1101–1107 (2006).
Dogan, A., Attygalle, A. D. & Kyriakou, C. Angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 121, 681–691 (2003).
Dorfman, D. M., Brown, J. A., Shahsafaei, A. & Freeman, G. J. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30, 802–810 (2006).
Rodriguez Pinilla, S. M. et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am. J. Surg. Pathol. 33, 81–90 (2009).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nature Immunol. 9, 1347–1355 (2008).
Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunol. 9, 1341–1346 (2008).
Acknowledgements
I would like to thank H. McGuire, A. Basten and S. Tangye for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Somatic hypermutation
-
A unique mutation mechanism that is targeted to the variable regions of rearranged immunoglobulin gene segments. Combined with the selection for B cells that produce high-affinity antibody, somatic hypermutation leads to affinity maturation of B cells in germinal centres.
- Class switch recombination
-
The process by which proliferating B cells rearrange their DNA to switch from expressing IgM (or another class of immunoglobulin) to expressing a different immunoglobulin heavy-chain constant region, thereby producing antibody with different effector functions.
- Asymmetrical cell division
-
A type of division that produces two daughter cells with different properties. This is in contrast to normal cell divisions, which give rise to equivalent daughter cells. Notably, stem cells can divide asymmetrically to give rise to two distinct daughter cells: one copy of themselves and one cell programmed to differentiate into another cell type.
- TH17 cell
-
A subset of CD4+ T helper cells that produce IL-17 and that are thought to be important in inflammatory and autoimmune diseases. Their generation involves IL-21 and IL-23, as well as the transcription factors RORγt and STAT3.
- Sanroque mice
-
An autoimmune strain of mouse that has a loss-of-function mutation in the gene roquin (also known as Rc3h1). These mice develop a T cell-mediated systemic lupus erythematosus-like syndrome and severe autoimmune diabetes when bred onto a susceptible genetic background.
- X-linked lymphoproliferative disease
-
Individuals with X-linked lymphoproliferative disease have complicated immune dysfunctions, often triggered by infection with Epstein–Barr virus. Many patients develop fatal B cell lymphoproliferation. The gene that encodes SAP is mutated in these patients.
- Follicular DCs
-
Specialized non-haematopoietic stromal cells that reside in the follicles and germinal centres. These cells have long dendrites, but are not related to dendritic cells, and carry intact antigen on their surface.
- IL-4-reporter mice
-
Genetically engineered knock-in mice in which the gene encoding IL-4 has been replaced by sequences that encode a reporter molecule, such as green fluorescent protein (GFP). When the IL-4 promoter region is activated, GFP is expressed and GFP+ cells can be seen by flow cytometry.
- Activation-induced cytidine deaminase
-
An enzyme that is required for two crucial events in the germinal centre: somatic hypermutation and class switch recombination.
- Experimental autoimmune encephalomyelitis
-
An experimental model for the human disease multiple sclerosis. Autoimmune disease is induced in experimental animals by immunization with myelin or peptides derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
Rights and permissions
About this article
Cite this article
King, C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol 9, 757–766 (2009). https://doi.org/10.1038/nri2644
Issue Date:
DOI: https://doi.org/10.1038/nri2644
This article is cited by
-
Comprehensive analysis of clinical, pathological, and genomic characteristics of follicular helper T-cell derived lymphomas
Experimental Hematology & Oncology (2021)
-
Src Family Protein Kinase Controls the Fate of B Cells in Autoimmune Diseases
Inflammation (2021)
-
Regulatory T Cell Plasticity and Stability and Autoimmune Diseases
Clinical Reviews in Allergy & Immunology (2020)
-
Early changes within the lymphocyte population are associated with the development of multiple organ dysfunction syndrome in trauma patients
Critical Care (2016)
-
Increased expression of transcription factor Bcl-6 in chronic rhinosinusitis with nasal polyps
European Archives of Oto-Rhino-Laryngology (2016)