Key Points
-
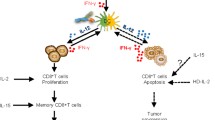
Interleukin-2 (IL-2) and IL-15 have both similar and contrasting functional roles in the life and death of lymphocytes.
-
The heterotrimeric receptors for these cytokines have two subunits in common: IL-2/15Rβ (also known as the IL-2 receptor β-chain (IL-2Rβ) and IL-15Rβ); and the common cytokine-receptor γ-chain (γc). The two cytokine receptors also have distinct α-subunits.
-
In many adaptive immune responses, IL-2 and IL-15 have distinct, and often competing, actions. IL-2 has a role in activation-induced cell death and in maintenance of regulatory T cells. In this way, it is involved in the elimination of self-reactive T cells, which if left unregulated could lead to the development of autoimmune diseases. By contrast, IL-15 is pivotal in the maintenance of long-lasting, high-avidity CD8+ memory T cells that are involved in the elimination of invading pathogens, thereby protecting the host against infection.
-
IL-2 is a secreted cytokine and binds pre-formed heterotrimeric receptors on the surface of activated cells. By contrast, IL-15 is mainly membrane bound, and it induces signalling in the context of cell–cell contact, at the immunological synapse. The unique subunit of the IL-15R, IL-15Rα, presents IL-15 in trans to neighbouring natural killer (NK) cells and CD8+ T cells.
-
IL-15 activates T cells and NK cells and has a role in persistence of CD8+ memory T cells. It therefore might be better than IL-2 for the treatment of cancer and as a component of molecular vaccines against infectious diseases.
-
Because IL-15 activates tumour-necrosis-factor expression and facilitates memory CD8+ T-cell maintenance, dysregulation of IL-15 is associated with a range of autoimmune inflammatory diseases. Therapeutic strategies that inhibit the actions of IL-15 are being developed for the treatment of T-cell-mediated autoimmune inflammatory diseases.
Abstract
Interleukin-2 and interleukin-15 have pivotal roles in the control of the life and death of lymphocytes. Although their heterotrimeric receptors have two receptor subunits in common, these two cytokines have contrasting roles in adaptive immune responses. The unique role of interleukin-2 is in the elimination of self-reactive T cells to prevent autoimmunity. By contrast, interleukin-15 is dedicated to the prolonged maintenance of memory T-cell responses to invading pathogens. As discussed in this Review, the biology of these cytokines will affect the development of novel therapies for malignancy and autoimmune diseases, as well as the design of vaccines against infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sugamura, K. et al. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 14, 179–205 (1996).
Noguchi, M. et al. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science 262, 1877–1880 (1993).
Waldmann, T. A., Dubois, S. & Tagaya, Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14, 105–110 (2001). This Review provides an analysis of the similarities and differences in the functions of IL-2 and IL-15, as well as the mechanisms that underlie the distinct functions.
Waldmann, T. A. & Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 (1999).
Fehniger, T. A. & Caligiuri, M. A. Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 (2001). This is an excellent review of IL-15 biology. It discusses the disorders of IL-15 in human diseases and considers the implications of these abnormalities for immunotherapy.
Bamford, R. N. et al. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl Acad. Sci. USA 91, 4940–4944 (1994).
Grabstein, K. H. et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264, 965–968 (1994).
Giri, J. G. et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 14, 3654–3663 (1995).
Waldmann, T. A. The interleukin-2 receptor. J. Biol. Chem. 266, 2681–2684 (1991).
Taniguchi, T. & Minami, Y. The IL-2/IL-2 receptor system: a current overview. Cell 73, 5–8 (1993).
Miyazaki, T. et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell 81, 223–231 (1995).
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998). This was the first report to indicate that IL-15 has an important role in stimulation of the proliferation of CD8+CD44hi memory T cells.
Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Marks-Konczalik, J. et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl Acad. Sci. USA 97, 11445–11450 (2000).
Fehniger, T. A., Cooper, M. A. & Caligiuri, M. A. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13, 169–183 (2002).
Carson, W. E. et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99, 937–943 (1997).
Lenardo, M. J. Fas and the art of lymphocyte maintenance. J. Exp. Med. 183, 721–724 (1996).
Fontenot, J. D., Rassmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin-2 in FOXP3-expressing regulatory T cells. Nature Immunol. 6, 1142–1151 (2005).
D'Cruz, L. M. & Klein, L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nature Immunol. 6, 1152–1159 (2005).
Maloy, K. J. & Powrie, F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nature Immunol. 6, 1071–1072 (2005).
Schluns, K. S., Klonowski, K. D. & Lefrancois, L. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood 103, 988–994 (2004).
Becker, T. C. et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 (2002).
Rosenberg, S. A. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J. Sci. Am. 6, S2–S7 (2000).
Waldmann, T. A. et al. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood 82, 1701–1712 (1993).
Morris, J. C. et al. Preclinical and Phase I clinical trial of blockade of IL-15 using Mikβ1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc. Natl Acad. Sci. USA 103, 401–406 (2006).
Waldmann, T. A., Dubois, S., Muller, J., Goldman, C. & Damjanovich, S. in Biophysical Aspects of Transmembrane Signaling (ed. Damjanovich, S.) 97–121 (Springer, Heidelberg, 2005).
Schorle, H., Holtschke, T., Hunig, T., Schimpl, A. & Horak, I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352, 621–624 (1991).
Sadlack, B. et al. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur. J. Immunol. 24, 281–284 (1994).
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Kishimoto, T. et al. (eds) Leukocyte typing VI. White Cell Differentiation Antigens (Garland, New York, 1997).
Dubois, S. et al. Distinct pathways involving the FK506-binding proteins 12 and 12.6 underlie IL-2 versus IL-15-mediated proliferation of cells. Proc. Natl Acad. Sci. USA 100, 14169–14174 (2003).
Bulfone-Paus, S. et al. Death deflected: IL-15 inhibits TNF-α-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J. 13, 1575–1585 (1999).
Damjanovich, S. et al. Preassembly of interleukin 2 (IL-2) receptor subunits on resting Kit 225 K6 T cells and their modulation by IL-2, IL-7, and IL-15: a fluorescence resonance energy transfer study. Proc. Natl Acad. Sci. USA 94, 13134–13139 (1997).
Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002). This was the first study to show that IL-15 bound to IL-15Rα recycles through endosomal vesicles and that IL-15Rα on the surface of monocytes and DCs presents IL-15 in trans to NK cells and CD8+CD44hi memory T cells. In this way, the study showed that IL-15 has an important role in the generation, persistence and differentiation of NK cells and CD8+CD44hi memory T cells.
Lodolce, J. P., Burkett, P. R., Boone, D. L., Chien, M. & Ma, A. T-cell-independent interleukin 15Rα signals are required for bystander proliferation. J. Exp. Med. 194, 1187–1194 (2001).
Rosenberg, S. A. et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA 271, 907–913 (1994).
Munger, W. et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell. Immunol. 165, 289–293 (1995).
Evans, R., Fuller, J. A., Christianson, G., Krupke, D. M. & Troutt, A. B. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell. Immunol. 179, 66–73 (1997).
Yajima, T. et al. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int. J. Cancer 99, 573–578 (2002).
Kobayashi, H. et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727 (2005).
Oh, S., Berzofsky, J. A., Burke, D. S., Waldmann, T. A. & Perera, L. P. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl Acad. Sci. USA 100, 3392–3397 (2003). In this study, vaccines that express IL-15 were found to induce long-lasting CD8+ T-cell-mediated immunity, whereas vaccines incorporating IL-2 elicited short-lived immunity.
Oh, S., Perera, L. P., Burke, D. S., Waldmann, T. A. & Berzofsky, J. A. IL-15/IL-15Rα-mediated avidity maturation of memory CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 15154–15159 (2004).
Kutzler, M. A. et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 175, 112–123 (2005).
Nashan, B. et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet 350, 1193–1198 (1997).
Kirkman, R. L. et al. A randomized prospective trial of anti-Tac monoclonal antibody in human renal transplantation. Transplantation 51, 107–113 (1991).
Vincenti, F. et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N. Engl. J. Med. 338, 161–165 (1998). This report of a Phase III clinical trial of daclizumab shows that it is effective for the reduction of acute renal-allograft rejection episodes in patients receiving a human renal transplant. On the basis of this study, the FDA approved daclizumab for use in renal organ-transplantation protocols.
Waldmann, T. A. et al. Radioimmunotherapy of interleukin-2Rα-expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood 86, 4063–4075 (1995).
Nussenblatt, R. B. et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a Phase I/II clinical trial. Proc. Natl Acad. Sci. USA 96, 7462–7466 (1999).
Bielekova, B. et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon β. Proc. Natl Acad. Sci. USA 101, 8705–8708 (2004). This paper shows that treatment with daclizumab leads to a 78% reduction in new contrast-enhancing lesions in patients with multiple sclerosis who have failed to respond to treatment with IFNβ.
Waldmann, T. A. et al. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sezary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J. Clin. Invest. 73, 1711–1718 (1984).
Waldmann, T. A. The meandering 45-year odyssey of a clinical immunologist. Annu. Rev. Immunol. 21, 1–27 (2002).
McInnes, I. B., Leung, B. P., Sturrock, R. D., Field, M. & Liew, F. Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nature Med. 3, 189–195 (1997). This study found that IL-15 effectively induces TNF production in patients with rheumatoid arthritis, through a process that requires the interaction of synovial cells and T cells.
Azimi, N., Nagai, M., Jacobson, S. & Waldmann, T. A. IL-15 plays a major role in the persistence of Tax-specific CD8 cells in HAM/TSP patients. Proc. Natl Acad. Sci. USA 98, 14559–14564 (2001).
Ruchatz, H., Leung, B. P., Wei, X. Q., McInnes, I. B. & Liew, F. Y. Soluble IL-15 receptor α-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 160, 5654–5660 (1998).
Kim, Y. S. et al. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fcγ2a protein blocks delayed-type hypersensitivity. J. Immunol. 160, 5742–5748 (1998).
Baslund, B. et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 52, 2686–2692 (2005).
Villadsen, L. S. et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112, 1571–1580 (2003).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Harada, S. et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 42, 1508–1516 (1999).
Oppenheimer-Marks, N., Brezinschek, R. I., Mohamadzadeh, M., Vita, R. & Lipsky, P. E. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse–human rheumatoid arthritis model in vivo. J. Clin. Invest. 101, 1261–1272 (1998).
Losy, J., Niezgoda, A. & Zaremba, J. IL-15 is elevated in sera of patients with relapsing–remitting multiple sclerosis. Folia Neuropathol. 40, 151–153 (2002).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Feldmann, M., Brennan, F. M. & Maini, R. N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14, 397–440 (1996).
Guex-Crosier, Y. et al. Humanized antibodies against the α-chain of the IL-2 receptor and against the β-chain shared by the IL-2 and IL-15 receptors in a monkey uveitis model of autoimmune diseases. J. Immunol. 158, 452–458 (1997).
Hakimi, J. et al. Humanized Mikβ1, a humanized antibody to the IL-2 receptor β-chain that acts synergistically with humanized anti-TAC. J. Immunol. 151, 1075–1085 (1993).
Tinubu, S. A. et al. Humanized antibody directed to the IL-2 receptor β-chain prolongs primate cardiac allograft survival. J. Immunol. 153, 4330–4338 (1994).
Leonard, W. J. in Cytokine Reference Vol. 2 (eds Oppenheim, J. J. & Feldmann, M.) 1439–1457 (Academic, San Diego, 2001).
Acknowledgements
This research was supported by the Intramural Program of the National Cancer Institute, National Institutes of Health (USA).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design Nature Reviews Immunology 6, 595–601 (2006); doi:10.1038/nri1901
Together with the US government, Thomas Waldmann holds patent number 5,833,983, entitled Interleukin-2 receptor (IL-2Rβ) and application thereof. This patent provides royalty payments.
Glossary
- Activation-induced cell death
-
(AICD). A process by which fully activated T cells undergo programmed cell death through engagement of cell-surface-expressed death receptors such as CD95 (also known as FAS) or the tumour-necrosis-factor receptor.
- CD4+CD25+ regulatory T cell
-
(TReg cell). A specialized type of CD4+ T cell that can suppress the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral self-tolerance and are characterized by expression of the α-chain of the interleukin-2 receptor (also known as CD25) and the transcription factor forkhead box P3 (FOXP3).
- Intestinal intraepithelial lymphocyte
-
A T cell that resides in the basolateral side of the intestinal epithelium. These cells express either an αβ-T-cell receptor (TCR) or a γδ-TCR, as well as a CD8αα homodimer. This is in contrast to most conventional CD8+ T cells, which express a CD8αβ heterodimer. CD8αα is a ligand for the non-classical MHC class I molecule thymus leukaemia antigen (TL), which is expressed by the intestinal epithelium.
- Trans-presentation
-
A process by which the α-chain of the interleukin-15 (IL-15) receptor (IL-15Rα) presents active IL-15 in trans to opposing cells expressing a complex (with an intermediate affinity for IL-15) that contains the β-chain IL-2/15Rβ and the common cytokine-receptor γ-chain (γc), thereby transducing a signal.
- Fluorescence resonance energy transfer
-
(FRET). A technique that is used to measure protein–protein interactions either by microscopy or flow cytometry. Proteins fused to cyan, yellow or red fluorescent proteins are expressed and assessed for interaction by measuring the energy transfer between fluorophores. Such transfer can only occur if proteins physically interact.
- Immunological synapse
-
A region that can form between two cells of the immune system in close contact. The name derives from similarities to the synapses that occur in the nervous system. The immunological synapse refers to the interaction between a T cell or natural killer cell and an antigen-presenting cell. This interface involves adhesion molecules, as well as antigen receptors and cytokine receptors.
- Delayed-type hypersensitivity
-
A cellular immune response to antigen that develops over 24–72 hours. It involves the infiltration of T cells and monocytes, and it depends on the production of T-helper-1 cytokines.
- Collagen-induced arthritis
-
An experimental model of rheumatoid arthritis. Arthritis is induced by immunization of susceptible animals with type II collagen.
Rights and permissions
About this article
Cite this article
Waldmann, T. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6, 595–601 (2006). https://doi.org/10.1038/nri1901
Issue Date:
DOI: https://doi.org/10.1038/nri1901
This article is cited by
-
Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis
Cell Death & Disease (2023)
-
Emerging principles of cytokine pharmacology and therapeutics
Nature Reviews Drug Discovery (2023)
-
Roles of natural killer cells in immunity to cancer, and applications to immunotherapy
Nature Reviews Immunology (2023)
-
Modulation of immune genes in the mucosal-associated lymphoid tissues of cobia by Sarcodia suae extract
Veterinary Research Communications (2023)
-
Therapeutic potential of FLT4-targeting peptide in acute myeloid leukemia
Cancer Immunology, Immunotherapy (2023)