Key Points
-
V(D)J recombination allows the vertebrate immune system to generate a diverse array of antigen receptors. Because this process introduces double-strand DNA breaks millions of times each day, it is potentially dangerous.
-
The first step in V(D)J recombination is site recognition and cleavage. The V(D)J recombinase creates a synaptic complex with two recombination signal sequences (RSSs), nicks the DNA between the RSS and the coding flank, and converts the nick to a double-stand break by forming a hairpin at the coding end.
-
The broken DNA ends are then ligated to form a coding joint (the new antigen-receptor gene) and a signal joint (which has no known immunological function).
-
There are three models for recombinase-mediated chromosome translocations: substrate-selection error, end donation and transposition.
-
Factors that help to prevent substrate-selection errors include: the V(D)J recombinase normally requires a pair of RSSs to carry out cleavage (coupled cleavage); chromatin accessibility to the V(D)J recombinase is carefully regulated; and the recombinase has a preference for intralocus recombination.
-
Defences against end donation include: classical non-homologous end joining (NHEJ) minimizes the potential for error (compared with the error-prone alternative NHEJ pathway); and the recombination-activating gene (RAG) post-cleavage complex seems to work closely with classical NHEJ.
-
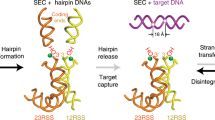
Defences against transposition include: non-standard products capitalize on the preference of RAG proteins to transpose into hairpin structures; and full-length RAG2 inhibits target capture in the presence of coding ends, and GTP also inhibits target capture.
Abstract
Chromosome breakage — a dangerous event that has triggered the evolution of several double-strand break repair pathways — has been co-opted by the immune system as an integral part of B- and T-cell development. This is a daring strategy, as improper repair can be deadly for the cell, if not for the whole organism. Even more daring, however, is the choice of a promiscuous transposase as the nuclease responsible for chromosome breakage, as the possibility of transposition brings an entirely new set of risks. What mechanisms constrain the dangerous potential of the recombinase and preserve genomic integrity during immune-system development?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shaffer, A. L., Rosenwald, A. & Staudt, L. M. Lymphoid malignancies: the dark side of B-cell differentiation. Nature Rev. Immunol. 2, 920–932 (2002).
Tycko, B. & Sklar, J. Chromosomal translocations in lymphoid neoplasia: a reappraisal of the recombinase model. Cancer Cells 2, 1–8 (1990).
Vanasse, G. J., Concannon, P. & Willerford, D. M. Regulated genomic instability and neoplasia in the lymphoid lineage. Blood 94, 3997–4010 (1999).
Roth, D. B. & Roth, S. Y. Unequal access: regulating V(D)J recombination through chromatin remodeling. Cell 103, 699–702 (2000).
Bassing, C. H., Swat, W. & Alt, F. W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109, S45–S55 (2002).
Lewis, S. M. The mechanism of V(D)J joining: lessons from molecular, immunological and comparative analyses. Adv. Immunol. 56, 27–150 (1994).
Hesse, J. E., Lieber, M. R., Mizuuchi, K. & Gellert, M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 3, 1053–1061 (1989).
Oettinger, M. A., Schatz, D. G., Gorka, C. & Baltimore, D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248, 1517–1523 (1990).
van Gent, D. C., Hiom, K., Paull, T. T. & Gellert, M. Stimulation of V(D)J cleavage by high mobility group proteins. EMBO J. 16, 2665–2670 (1997).
Silver, D. P., Spanopoulou, E., Mulligan, R. C. & Baltimore, D. Dispensable sequence motifs in the RAG-1 and RAG-2 genes for plasmid V(D)J recombination. Proc. Natl Acad. Sci. USA 90, 6100–6104 (1993).
Sadofsky, M. J., Hesse, J. E. & Gellert, M. Definition of a core region of RAG-2 that is functional in V(D)J recombination. Nucleic Acids Res. 22, 1805–1809 (1994).
Sadofsky, M. J., Hesse, J. E., McBlane, J. F. & Gellert, M. Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 22, 550 (1994).
Cuomo, C. A. & Oettinger, M. A. Analysis of regions of RAG-2 important for V(D)J recombination. Nucleic Acids Res. 22, 1810–1814 (1994).
Kirch, S. A., Sudarsanam, P. & Oettinger, M. A. Regions of RAG1 protein critical for V(D)J recombination. Eur. J. Immunol. 26, 886–891 (1996).
Roth, D. B., Menetski, J. P., Nakajima, P. B., Bosma, M. J. & Gellert, M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell 70, 983–991 (1992).
Roth, D. B., Zhu, C. & Gellert, M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc. Natl Acad. Sci. USA 90, 10788–10792 (1993).
Schlissel, M., Constantinescu, A., Morrow, T., Baxter, M. & Peng, A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 7, 2520–2532 (1993).
McBlane, J. F. et al. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell 83, 387–395 (1995).
Sadofsky, M. J. The RAG proteins in V(D)J recombination: more than just a nuclease. Nucleic Acids Res. 29, 1399–1409 (2001).
Gellert, M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71, 101–132 (2002).
van Gent, D. C., Mizuuchi, K. & Gellert, M. Similarities between initiation of V(D)J recombination and retroviral integration. Science 271, 1592–1594 (1996).
Roth, D. B. & Craig, N. L. VDJ recombination: a transposase goes to work. Cell 94, 411–414 (1998).
Kennedy, A. K., Guhathakurta, A., Kleckner, N. & Haniford, D. B. Tn10 transposition via a DNA hairpin intermediate. Cell 95, 125–134 (1998).
Kennedy, A. K., Haniford, D. B. & Mizuuchi, K. Single active site catalysis of the successive phosphoryl transfer steps by DNA transposases: insights from phosphorothioate stereoselectivity. Cell 101, 295–305 (2000).
Davies, D. R., Goryshin, I. Y., Reznikoff, W. S. & Rayment, I. Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. Science 289, 77–85 (2000).
Coen, E., Robbins, T. P., Almeida, J., Hudson, A. & Carpenter, R. in Mobile DNA (eds Berg, D. E. & Howe, M. M.) 413–436 (ASM Press, Washington DC, 1989).
Weil, C. F. & Kunze, R. Transposition of maize Ac/Ds transposable elements in the yeast Saccharomyces cerevisiae. Nature Genet. 26, 187–190 (2000).
Kunze, R. & Weil, C. F. in Mobile DNA II (ed. Craig, N. L.) 565–610 (ASM Press, Washington DC, 2002).
Ramsden, D. A., McBlane, J. F., van Gent, D. C. & Gellert, M. Distinct DNA sequence and structure requirements for the two steps of V(D)J recombination signal cleavage. EMBO J. 15, 3197–3206 (1996).
Eastman, Q. M. & Schatz, D. G. Nicking is asynchronous and stimulated by synapsis in 12/23 rule-regulated V(D)J cleavage. Nucleic Acids Res. 25, 4370–4378 (1997).
van Gent, D. C., Ramsden, D. A. & Gellert, M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell 85, 107–113 (1996).
Yu, K. & Lieber, M. R. The nicking step in V(D)J recombination is independent of synapsis: implications for the immune repertoire. Mol. Cell. Biol. 20, 7914–7921 (2000).
Steen, S. B., Gomelsky, L. & Roth, D. B. The 12/23 rule is enforced at the cleavage step of V(D)J recombination in vivo. Genes Cells 1, 543–553 (1996).
Eastman, Q. M., Leu, T. M. J. & Schatz, D. G. Initiation of V(D)J recombination in vitro: obeying the 12/23 rule. Nature 380, 85–88 (1996).
Bassing, C. H. et al. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature 405, 583–586 (2000).
Tillman, R. E. et al. Cutting edge: targeting of Vβ to Dβ rearrangement by RSSs can be mediated by the V(D)J recombinase in the absence of additional lymphoid-specific factors. J. Immunol. 170, 5–9 (2003).
Jung, D. et al. Extrachromosomal recombination substrates recapitulate beyond 12/23 restricted VDJ recombination in nonlymphoid cells. Immunity 18, 65–74 (2003).
Lewis, S. M., Hesse, J. E., Mizuuchi, K. & Gellert, M. Novel strand exchanges in V(D)J recombination. Cell 55, 1099–1107 (1988).
Morzycka-Wroblewska, E., Lee, F. & Desiderio, S. Unusual immunoglobulin gene rearrangement leads to replacement of recombination signal sequences. Science 242, 261–263 (1988).
Carroll, A. M., Slack, J. K. & Mu, X. V(D)J recombination generates a high frequency of nonstandard TCRδ-associated rearrangements in thymocytes. J. Immunol. 150, 2222–2230 (1993).
Bogue, M. A., Wang, C., Zhu, C. & Roth, D. B. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal and hybrid joint formation. Immunity 7, 37–47 (1997).
Sollbach, A. E. & Wu, G. E. Inversions produced during V(D)J rearrangement at IgH, the immunoglobulin heavy-chain locus. Mol. Cell. Biol. 15, 671–681 (1995).
VanDyk, L. F., Wise, T. W., Moore, B. B. & Meek, K. Immunoglobulin DH recombination signal sequence targeting. J. Immunol. 157, 4005–4015 (1996).
Grawunder, U. & Harfst, E. How to make ends meet in V(D)J recombination. Curr. Opin. Immunol. 13, 186–194 (2001).
Dai, Y. et al. Nonhomologous end joining and V(D)J recombination require an additional factor. Proc. Natl Acad. Sci. USA 100, 2462–2467 (2003).
Walker, J. R., Corpina, R. A. & Goldberg, J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412, 607–614 (2001).
Mahajan, K. N. et al. Association of terminal deoxynucleotidyl transferase with Ku. Proc. Natl Acad. Sci. USA 96, 13926–13931 (1999).
Purugganan, M. M., Shah, S., Kearney, J. F. & Roth, D. B. Ku80 is required for addition of N nucleotides to V(D)J recombination junctions by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 29, 1638–1646 (2001).
Zhu, C., Bogue, M. A., Lim, D. -S., Hasty, P. & Roth, D. B. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell 86, 379–389 (1996).
Leber, R., Wise, T. W., Mizuta, R. & Meek, K. The XRCC4 gene product is a target for and interacts with the DNA-dependent protein kinase. J. Biol. Chem. 273, 1794–1801 (1998).
Ma, Y., Pannicke, U., Schwarz, K. & Lieber, M. R. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108, 781–794 (2002). This paper describes biochemical evidence that Artemis opens hairpin coding ends and provides a mechanistic link between Artemis, hairpin opening and DNA-dependent protein kinase (DNA-PK).
Mahajan, K. N., Nick McElhinny, S. A., Mitchell, B. S. & Ramsden, D. A. Association of DNA polymerase μ (pol μ) with Ku and ligase IV: role for pol mu in end-joining double-strand break repair. Mol. Cell. Biol. 22, 5194–5202 (2002).
Critchlow, S. E., Bowater, R. P. & Jackson, S. P. Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Curr. Biol. 7, 588–598 (1997).
Grawunder, U. et al. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature 388, 492–495 (1997).
Modesti, M., Hesse, J. E. & Gellert, M. DNA binding of XRCC4 protein is associated with V(D)J recombination but not with stimulation of DNA ligase IV activity. EMBO J. 18, 2008–2018 (1999).
Qiu, J. X., Kale, S. B., Yarnell Schultz, H. & Roth, D. B. Separation-of-function mutants reveal critical roles for RAG2 in both the cleavage and joining steps of V(D)J recombination. Mol. Cell 7, 77–87 (2001).
Yarnall Schultz, H., Landree, M. A., Qiu, J. X., Kale, S. B. & Roth, D. B. Joining-deficient RAG1 mutants block V(D)J recombination in vivo and hairpin opening in vitro. Mol. Cell 7, 65–75 (2001). Separation-of-function mutants in recombination-activating gene 1 ( RAG1 ) in this and reference 58 provided the first evidence that the RAG post-cleavage complex is crucial for joining, functioning as a scaffold for both coding and signal ends in living cells.
Huye, L. E., Purugganan, M. M., Jiang, M. M. & Roth, D. B. Mutational analysis of all conserved basic amino acids in RAG-1 reveals catalytic, step arrest, and joining-deficient mutants in the V(D)J recombinase. Mol. Cell. Biol. 22, 3460–3473 (2002). Cells that express joining-deficient RAG1 mutants produce joints with the same kinds of abnormalities that are seen in non-homologous end joining (NHEJ)-deficient mutants, providing evidence that the RAG proteins actively collaborate with the classical NHEJ pathway.
Rooney, S. et al. Leaky scid phenotype associated with defective V(D)J coding end processing in Artemis-deficient mice. Mol. Cell 10, 1379–1390 (2002). An Artemis-knockout mouse confirms that Artemis does indeed have an important role in opening hairpin-coding ends, but the leaky phenotype of these mice hints that alternative pathways might also carry out this function. This paper also shows that Artemis deficiency leads to genomic instability in fibroblasts.
Besmer, E. et al. Hairpin coding end opening is mediated by RAG1 and RAG2 proteins. Mol. Cell 2, 817–828 (1998).
Shockett, P. E. & Schatz, D. G. DNA hairpin opening mediated by the RAG1 and RAG2 proteins. Mol. Cell. Biol. 19, 4159–4166 (1999).
Paull, T. T. & Gellert, M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell 1, 969–979 (1998).
Mansilla-Soto, J. & Cortes, P. VDJ recombination: Artemis and its in vivo role in hairpin opening. J. Exp. Med. 197, 543–547 (2003).
Yurchenko, V., Xue, Z. & Sadofsky, M. The RAG1 N-terminal domain is an E3 ubiquitin ligase. Genes Dev. 17, 581–585 (2003).
Vanasse, G. J. et al. Genetic pathway to recurrent chromosome translocations in murine lymphoma involves V(D)J recombinase. J. Clin. Invest. 103, 1669–1675 (1999).
Kuppers, R. & Dalla-Favera, R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene 20, 5580–5594 (2001).
Kirsch, I. R., Morton, C. C., Nakahara, K. & Leder, P. Human immunoglobulin heavy chain genes map to a region of translocations in malignant B lymphocytes. Science 216, 301–303 (1982).
Dalla-Favera, R. et al. Human c-myc oncogene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc. Natl Acad. Sci. USA 79, 7824–7827 (1982).
Tycko, B., Palmer, J. D. & Sklar, J. T cell receptor gene trans-rearrangements: chimeric γδ genes in normal lymphoid tissues. Science 245, 1242–1246 (1989).
Tycko, B., Coyle, H. & Sklar, J. Chimeric γδ signal joints: implications for the mechanism and regulation of T cell receptor gene rearrangement. J. Immunol. 147, 705–713 (1991).
Bailey, S. N. & Rosenberg, N. Assessing the pathogenic potential of the V(D)J recombinase by interlocus immunoglobulin light-chain gene rearrangement. Mol. Cell. Biol. 17, 887–894 (1997).
Kobayashi, Y., Tycko, B., Soreng, A. L. & Sklar, J. Transrearrangements between antigen receptor genes in normal human lymphoid tissues and in ataxia telangiectasia. J. Immunol. 147, 3201–3209 (1991).
Lipkowitz, S., Stern, M. H. & Kirsch, I. R. Hybrid T cell receptor genes formed by interlocus recombination in normal and ataxia-telangiectasia lymphocytes. J. Exp. Med. 172, 409–418 (1990).
Kirsch, I. R. & Lipkowitz, S. A measure of genomic instability and its relevance to lymphomagenesis. Cancer Res. 52, 5545s–5546s (1992).
Steen, S. B., Gomelsky, L., Speidel, S. L. & Roth, D. B. Initiation of V(D)J recombination in vivo: role of recombination signal sequences in formation of single and paired double-strand breaks. EMBO J. 16, 2656–2664 (1997).
Bakhshi, A. et al. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc. Natl Acad. Sci. USA 84, 2396–2400 (1987). The initial description of the end-donation model for RAG-mediated chromosome translocations. Evidence that this is a common mechanism for lymphomagenesis is also provided in references 77 and 78.
Welzel, N. et al. Templated nucleotide addition and immunoglobulin JH-gene utilization in t(11;14) junctions: implications for the mechanism of translocation and the origin of mantle cell lymphoma. Cancer Res. 61, 1629–1636 (2001).
Jager, U. et al. Follicular lymphomas' BCL-2/IgH junctions contain templated nucleotide insertions: novel insights into the mechanism of t(14;18) translocation. Blood 95, 3520–3529 (2000).
Han, J. -O., Steen, S. B. & Roth, D. B. Ku86 is not required for protection of signal ends or for formation of nonstandard V(D)J recombination products. Mol. Cell. Biol. 17, 2226–2234 (1997).
Han, J. -O., Erskine, L. A., Purugganan, M. M., Stamato, T. D. & Roth, D. B. V(D)J recombination intermediates and non-standard products in XRCC4-deficient cells. Nucleic Acids Res. 26, 3769–3775 (1998).
Melek, M., Gellert, M. & van Gent, D. C. Rejoining of DNA by the RAG1 and RAG2 proteins. Science 280, 301–303 (1998).
Agrawal, A., Eastman, Q. M. & Schatz, D. G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 394, 744–751 (1998).
Hiom, K., Melek, M. & Gellert, M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 94, 463–470 (1998). These authors show that the RAG proteins are capable of transposition in the test tube, and they propose several models for transposition-mediated genome rearrangements.
Thompson, C. B. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity 3, 531–539 (1995).
Messier, T. L., O'Neill, J. P., Hou, S. M., Nicklas, J. A. & Finette, B. A. In vivo transposition mediated by V(D)J recombinase in human T lymphocytes. EMBO J. 22, 1381–1388 (2003). The first reported evidence for RAG-mediated transposition in vivo.
Lee, G. S., Neiditch, M. B., Sinden, R. R. & Roth, D. B. Targeted transposition by the V(D)J recombinase. Mol. Cell. Biol. 22, 2068–2077 (2002). This work shows that RAG-mediated transposition is strongly targeted to hairpin ends and provides evidence that nonstandard junctions (hybrid and open-and-shut joints) can form by transposition, indicating that the formation of these products in vivo might function as a safety mechanism to prevent more dangerous transposition events.
Roth, D. B., Nakajima, P. B., Menetski, J. P., Bosma, M. J. & Gellert, M. V(D)J recombination in mouse thymocytes: double-strand breaks near T cell receptor δ rearrangement signals. Cell 69, 41–53 (1992).
Tillman, R. E. et al. Restrictions limiting the generation of DNA double strand breaks during chromosomal V(D)J recombination. J. Exp. Med. 195, 309–316 (2002).
Kosak, S. T. et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296, 158–162 (2002).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Taylor, A. M., Metcalfe, J. A., Thick, J. & Mak, Y. F. Leukemia and lymphoma in ataxia telangiectasia. Blood 87, 423–438 (1996).
Xu, Y. et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10, 2411–2422 (1996).
Liyanage, M. et al. Abnormal rearrangement within the αδ T-cell receptor locus in lymphomas from Atm-deficient mice. Blood 96, 1940–1946 (2000).
Lista, F., Bertness, V., Guidos, C. J., Danska, J. S. & Kirsch, I. R. The absolute number of trans-rearrangements between the TCR γ and TCR β loci is predictive of lymphoma risk: a severe combined immune deficiency (SCID) murine model. Cancer Res. 57, 4408–4413 (1997).
Perkins, E. J. et al. Sensing of intermediates in V(D)J recombination by ATM. Genes Dev. 16, 159–164 (2002).
Han, J. -O., Steen, S. B. & Roth, D. B. Intermolecular V(D)J recombination is prohibited specifically at the joining step. Mol. Cell 3, 331–338 (1999).
Tevelev, A. & Schatz, D. G. Intermolecular V(D)J recombination. J. Biol. Chem. 275, 8341–8348 (2000).
Hempel, W. M. et al. Enhancer control of V(D)J recombination at the TCR β locus: differential effects on DNA cleavage and joining. Genes Dev. 12, 2305–2317 (1998).
Takata, M. et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO 17, 5497–5508 (1998).
Paques, F. & Haber, J. E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63, 349–404 (1999).
Cox, M. M. et al. The importance of repairing stalled replication forks. Nature 404, 37–41 (2000).
Ferguson, D. O. & Alt, F. W. DNA double strand break repair and chromosomal translocation: lessons from animal models. Oncogene 20, 5572–5579 (2001).
Zhu, C. et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell 109, 811–821 (2002). Complex chromosomal rearrangements and gene amplification occur in mice that are doubly deficient for classic NHEJ and p53, implicating alternative, microhomology-mediated joining pathways.
Jackson, S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696 (2002).
Ferguson, D. O. et al. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc. Natl Acad. Sci. USA 97, 6630–6633 (2000).
Jhappan, C., Morse, H. C., Fleischmann, R. D., Gottesman, M. M. & Merlino, G. DNA-PKcs: a T-cell tumour suppressor encoded at the mouse scid locus. Nature Genet. 17, 483–486 (1997).
Custer, R. P., Bosma, G. C. & Bosma, M. J. Severe combined immunodeficiency in the mouse: pathology, reconstitution, neoplasms. Am. J. Pathol. 120, 464–477 (1985).
Gu, Y. et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity 7, 653–665 (1997).
Li, G. C. et al. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol. Cell 2, 1–8 (1998).
Difilippantonio, M. J. et al. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature 404, 510–514 (2000).
Gao, Y. et al. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 404, 897–900 (2000).
Moshous, D. et al. Partial T and B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. J. Clin. Invest. 111, 381–387 (2003). Artemis mutations predispose to lymphoma in humans, indicating that Artemis functions as a genome guardian.
Gladdy, R. A. et al. The RAG-1/2 endonuclease causes genomic instability and controls CNS complications of lymphoblastic leukemia in p53/Prkdc-deficient mice. Cancer Cell 3, 37–50 (2003).
Sharpless, N. E. et al. Impaired nonhomologous end-joining provokes soft tissue sarcomas harboring chromosomal translocations, amplifications, and deletions. Mol. Cell 8, 1187–1196 (2001).
Williams, C. J. et al. Irradiation promotes V(D)J joining and RAG-dependent neoplastic transformation in SCID T-cell precursors. Mol. Cell. Biol. 21, 400–413 (2001).
Danska, J. S. et al. Rescue of T cell-specific V(D)J recombination in SCID mice by DNA damaging agents. Science 266, 450–455 (1994).
Murphy, W. J. et al. Induction of T cell differentiation and lymphomagenesis in the thymus of mice with severe combined immunodeficiency (SCID). J. Immunol. 153, 1004–1014 (1994).
Wilson, J. H., Berget, P. B. & Pipas, J. M. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol. Cell. Biol. 2, 1258–1269 (1982).
Roth, D. B., Porter, T. N. & Wilson, J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol. Cell. Biol. 5, 2599–2607 (1985).
Roth, D. B. & Wilson, J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol. Cell. Biol. 6, 4295–4304 (1986).
Kabotyanski, E. B., Gomelsky, L., Han, J. -O., Stamato, T. D. & Roth, D. B. Double-strand break repair in Ku86- and XRCC4-deficient cells. Nucleic Acids Res. 26, 5333–5342 (1998).
Baumann, P. & West, S. C. DNA end-joining catalyzed by human cell-free extracts. Proc. Natl Acad. Sci. USA 95, 14066–14070 (1998).
Roth, D. B. Amplifying mechanisms of lymphomagenesis. Mol. Cell 10, 1–2 (2002).
Verkaik, N. S. et al. Different types of V(D)J recombination and end-joining defects in DNA double-strand break repair mutant mammalian cells. Eur. J. Immunol. 32, 701–709 (2002).
Agrawal, A. & Schatz, D. G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell 89, 43–53 (1997).
Hiom, K. & Gellert, M. Assembly of a 12/23 paired signal complex: a critical control point in V(D)J recombination. Mol. Cell 1, 1011–1019 (1998).
Jones, J. M. & Gellert, M. Intermediates in V(D)J recombination: a stable RAG1/2 complex sequesters cleaved RSS ends. Proc. Natl Acad. Sci. USA 98, 12926–12931 (2001).
Ramsden, D. A., Paull, T. T. & Gellert, M. Cell-free V(D)J recombination. Nature 388, 488–491 (1997).
Brandt, V. L. & Roth, D. B. A recombinase diversified: new functions of the RAG proteins. Curr. Opin. Immunol. 14, 224–229 (2002).
Tsai, C. L., Drejer, A. H. & Schatz, D. G. Evidence of a critical architectural function for the RAG proteins in end processing, protection, and joining in V(D)J recombination. Genes Dev. 16, 1934–1949 (2002).
Neiditch, M. B., Lee, G. S., Huye, L. E., Brandt, V. L. & Roth, D. B. The V(D)J recombinase efficiently cleaves and transposes signal joints. Mol. Cell 9, 871–878 (2002).
Zhu, C. & Roth, D. B. Characterization of coding ends in thymocytes of scid mice: implications for the mechanism of V(D)J recombination. Immunity 2, 101–112 (1995).
Marculescu, R. et al. Distinct t(7;9)(q34;q32) breakpoints in healthy individuals and individuals with T-ALL. Nature Genet. 33, 342–344 (2003). This work implicates signal joints in oncogenic chromosome translocations; see also reference 131.
Melek, M. & Gellert, M. RAG1/2-mediated resolution of transposition intermediates: two pathways and possible consequences. Cell 101, 625–633 (2000).
Sekiguchi, J. A., Whitlow, S. & Alt, F. W. Increased accumulation of hybrid V(D)J joins in cells expressing truncated versus full-length RAGs. Mol. Cell 8, 1383–1390 (2001).
Elkin, S. K., Matthews, A. G. & Oettinger, M. A. The C-terminal portion of RAG2 protects against transposition in vitro. EMBO J. 22, 1931–1938 (2003). This paper shows that the carboxyl terminus of RAG2 helps to prevent transposition in vitro in the presence of coding ends.
Tsai, C. L. & Schatz, D. G. Regulation of RAG1/RAG2-mediated transposition by GTP and the C-terminal region of RAG2. EMBO J. 22, 1922–1930 (2003). More evidence that the carboxyl terminus of RAG2 downregulates transposition in vitro ; furthermore, the authors find that GTP modulates transposition activity in vitro.
Livak, F. & Schatz, D. G. T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 16, 609–618 (1996).
Acknowledgements
I am grateful to S. Lewis, J. Petrini, M. Schlissel, G. Wu, K. Meek, N. Craig, G. Lee and V. Brandt for helpful discussions. I also thank G. Lee for providing figure 2. Work in my laboratory is supported by grants from the National Institutes of Health and by the Irene Diamond Foundation.
Author information
Authors and Affiliations
Glossary
- TRANSPOSASE
-
A protein that carries out transposition — that is, moves a segment of DNA from one position in the genome to another position (or to a different genome).
- NON-HOMOLOGOUS END JOINING
-
(NHEJ). One of the two main double-strand break repair pathways, the other being homologous recombination. An 'alternative NHEJ pathway' that does not depend on the six known NHEJ factors (Ku70, Ku86, DNA-PKcs, XRCC4, Artemis and DNA ligase IV) has been described, but it is not well understood.
Rights and permissions
About this article
Cite this article
Roth, D. Restraining the V(D)J recombinase. Nat Rev Immunol 3, 656–666 (2003). https://doi.org/10.1038/nri1152
Issue Date:
DOI: https://doi.org/10.1038/nri1152
This article is cited by
-
RAG2 and XLF/Cernunnos interplay reveals a novel role for the RAG complex in DNA repair
Nature Communications (2016)
-
Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults
Nature Genetics (2016)
-
Generation and repair of AID-initiated DNA lesions in B lymphocytes
Frontiers of Medicine (2014)
-
Erratum to: The role of activation-induced deaminase in antibody diversification and genomic instability
Immunologic Research (2014)
-
The role of activation-induced deaminase in antibody diversification and genomic instability
Immunologic Research (2013)