Key Points
-
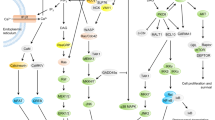
Five interactions between the tumour-necrosis factor receptor (TNFR)–TNF family members have emerged as positive regulators of T cells: OX40–OX40 ligand (OX40L), 4-1BB–4-1BBL, CD27–CD70, herpes-virus entry mediator (HVEM)–LIGHT and CD30–CD30L. The co-stimulatory TNFR-family members are mainly expressed by T cells and their ligands by antigen-presenting cells (APCs).
-
There are two patterns of expression of the co-stimulatory TNFR-family molecules: constitutive (such as CD27 and HVEM) and inducible (such as OX40, 4-1BB and CD30). All can potentially participate in T-cell responses either at the time of T-cell activation or within the first few days of activation.
-
In vitro and in vivo data indicate that HVEM–LIGHT interactions promote the early activation and clonal expansion of T cells, CD27–CD70 might also help to maintain early proliferation, OX40–OX40L and 4-1BB–4-1BBL and CD30–CD30L seem to regulate later expansion of T-cell numbers at the peak of the response.
-
Co-stimulatory TNFR-family signalling seems to regulate T-cell division, survival and effector function by activating nuclear factor-κB (NF-κB), JNK pathways and phosphatidylinositol 3-kinase (PI3K)–protein kinase B (PKB) pathways.
-
It is not clear whether all the TNFR-family molecules are required to function together (simultaneously or sequentially) to regulate a response or whether different responses require different co-stimulatory interactions.
-
Survival signals from TNFR-family members might be crucial for secondary responses and the maintenance of T-cell memory.
-
Blocking co-stimulatory TNF family members might be of benefit in inflammatory and autoimmune diseases and in preventing transplant rejection, whereas promoting these interactions might enhance antitumour immunity.
Abstract
Interactions between co-stimulatory ligands and their receptors are crucial for the activation of T cells, the prevention of tolerance and the development of T-cell immunity. It is now evident that members of the immunoglobulin-like CD28–B7 co-stimulatory family cannot fully account for an effective long-lasting T-cell response or the generation of memory T cells. Several members of the tumour-necrosis factor receptor (TNFR) superfamily — OX40, 4-1BB, CD27, CD30 and HVEM (herpes-virus entry mediator) — are poised to deliver co-stimulatory signals both early and late after encounter with antigen. The roles of these molecules in initiating and sustaining the T-cell response and in promoting long-lived immunity are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bodmer, J. L., Schneider, P. & Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Locksley, R. M., Killeen, N. & Lenardo, M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Borst, J. et al. Alternative molecular form of human T cell-specific antigen CD27 expressed upon T cell activation. Eur. J. Immunol. 19, 357–364 (1989).
Morel, Y. et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J. Immunol. 165, 4397–4404 (2000).
Gramaglia, I., Weinberg, A. D., Lemon, M. & Croft, M. Ox-40 ligand: a potent co-stimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161, 6510–6517 (1998).
Pollok, K. E., Kim, S. H. & Kwon, B. S. Regulation of 4-1BB expression by cell–cell interactions and the cytokines, interleukin-2 and interleukin-4. Eur. J. Immunol. 25, 488–494 (1995).
Ellis, T. M., Simms, P. E., Slivnick, D. J., Jack, H. M. & Fisher, R. I. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J. Immunol. 151, 2380–2389 (1993).
de Jong, R. et al. Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. J. Immunol. 146, 2488–2494 (1991).
Gravestein, L. A. et al. Cloning and expression of murine CD27: comparison with 4-1BB, another lymphocyte-specific member of the nerve growth factor receptor family. Eur. J. Immunol. 23, 943–950 (1993).
Lens, S. M., Baars, P. A., Hooibrink, B., van Oers, M. H. & van Lier, R. A. Antigen-presenting cell-derived signals determine expression levels of CD70 on primed T cells. Immunol. 90, 38–45 (1997).
Mauri, D. N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin-α are ligands for herpesvirus entry mediator. Immunity 8, 21–30 (1998).
Wiley, S. R., Goodwin, R. G. & Smith, C. A. Reverse signaling via CD30 ligand. J. Immunol. 157, 3635–3639 (1996).
Baum, P. R. et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 13, 3992–4001 (1994).
Gilfillan, M. C., Noel, P. J., Podack, E. R., Reiner, S. L. & Thompson, C. B. Expression of the co-stimulatory receptor CD30 is regulated by both CD28 and cytokines. J. Immunol. 160, 2180–2187 (1998).
Walker, L. S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190, 1115–1122 (1999).
Rogers, P. R., Song, J., Gramaglia, I., Killeen, N. & Croft, M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 15, 445–455 (2001). Using knockout T cells, this paper defines the sequential action of OX40 after CD28, and shows that OX40 controls T-cell survival through regulating anti-apoptotic BCL2-family members.
Diehl, L. et al. In vivo triggering through 4-1BB enables TH-independent priming of CTL in the presence of an intact CD28 co-stimulatory pathway. J. Immunol. 168, 3755–3762 (2002).
Lens, S. M. et al. Phenotype and function of human B cells expressing CD70 (CD27 ligand). Eur. J. Immunol . 26, 2964–2971 (1996).
Pollok, K. E. et al. 4-1BB T-cell antigen binds to mature B cells and macrophages, and co-stimulates anti-μ-primed splenic B cells. Eur. J. Immunol. 24, 367–374 (1994).
Ohshima, Y. et al. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 159, 3838–3848 (1997).
Shanebeck, K. D. et al. Regulation of murine B cell growth and differentiation by CD30 ligand. Eur. J. Immunol. 25, 2147–2153 (1995).
Hintzen, R. Q. et al. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J. Immunol. 152, 1762–1773 (1994).
Tamada, K. et al. LIGHT, a TNF-like molecule, co-stimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J. Immunol. 164, 4105–4110 (2000).
Oshima, H. et al. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 10, 517–526 (1998).
Futagawa, T. et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14, 275–286 (2002).
Kwon, B. S. et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 272, 14272–14276 (1997).
Harrop, J. A. et al. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J. Immunol. 161, 1786–1794 (1998).
Tamada, K. et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nature Med. 6, 283–289 (2000). The authors show that LIGHT has potent co-stimulatory activity, with an important role in graft-versus-host disease and can promote antitumour responses.
Ye, Q. et al. Modulation of LIGHT–HVEM co-stimulation prolongs cardiac allograft survival. J. Exp. Med. 195, 795–800 (2002).
Scheu, S. et al. Targeted disruption of LIGHT causes defects in co-stimulatory T cell activation and reveals cooperation with lymphotoxin-β in mesenteric lymph node genesis. J. Exp. Med. 195, 1613–1624 (2002).
Shaikh, R. B. et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J. Immunol. 167, 6330–6337 (2001).
Wang, J. et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J. Clin. Invest. 108, 1771–1780 (2001).
Tamada, K. et al. Cutting edge: selective impairment of CD8+ T cell function in mice lacking the TNF superfamily member LIGHT. J. Immunol. 168, 4832–4835 (2002).
Wan, X. et al. A TNF family member LIGHT transduces co-stimulatory signals into human T cells. J. Immunol. 169, 6813–6821 (2002).
Agematsu, K. et al. Role of CD27 in T cell immune response. Analysis by recombinant soluble CD27. J. Immunol. 153, 1421–1429 (1994).
Hintzen, R. Q. et al. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 154, 2612–2623 (1995).
Hendriks, J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 1, 433–440 (2000). This is the first description of CD27-deficient mice and shows that CD27 is essential for primary and secondary influenza-specific T-cell responses.
Arens, R. et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNγ-mediated B cell depletion. Immunity 15, 801–812 (2001).
Couderc, B. et al. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) co-stimulatory molecules in tumor cells. Cancer Gene Ther. 5, 163–175 (1998).
Lorenz, M. G., Kantor, J. A., Schlom, J. & Hodge, J. W. Anti-tumor immunity elicited by a recombinant vaccinia virus expressing CD70 (CD27L). Hum. Gene Ther. 10, 1095–1103 (1999).
Chen, A. I. et al. Ox40-ligand has a critical co-stimulatory role in dendritic cell–T cell interactions. Immunity 11, 689–698 (1999).
Kopf, M. et al. OX40-deficient mice are defective in TH cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity 11, 699–708 (1999).
Murata, K. et al. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 191, 365–374 (2000).
Gramaglia, I. et al. The OX40 co-stimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 165, 3043–3050 (2000). This paper shows that OX40 is essential for the generation of a high frequency of memory CD4+ T cells.
DeBenedette, M. A. et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 163, 4833–4841 (1999).
Tan, J. T., Whitmire, J. K., Ahmed, R., Pearson, T. C. & Larsen, C. P. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 163, 4859–4868 (1999).
Tan, J. T. et al. 4-1BB co-stimulation is required for protective anti-viral immunity after peptide vaccination. J. Immunol. 164, 2320–2325 (2000). This paper, together with references 45 and 46, shows that 4-1BB has an important role in primary and secondary CD8+ T-cell responses to viruses and virus peptides.
Cooper, D., Bansal-Pakala, P. & Croft, M. 41BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. Eur. J. Immunol. 32, 521–529 (2002).
Brocker, T. et al. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 29, 1610–1616 (1999).
Murata, K. et al. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J. Immunol. 169, 4628–4636 (2002).
Bansal-Pakala, P., Gebre-Hiwot Jember, A. & Croft, M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nature Med. 7, 907–912 (2001). The authors report that signalling through OX40 can prevent and reverse T-cell tolerance that is induced by encounter with soluble antigen in the absence of adjuvant.
Maxwell, J., Weinberg, A. D., Prell, R. A. & Vella, A. T. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J. Immunol. 164, 107–112 (2000).
Weatherill, A. R., Maxwell, J. R., Takahashi, C., Weinberg, A. D. & Vella, A. T. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell. Immunol. 209, 63–75 (2001).
De Smedt, T. et al. OX40 co-stimulation enhances the development of T cell responses induced by dendritic cells in vivo. J. Immunol. 168, 661–670 (2002).
Melero, I. et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nature Med. 3, 682–685 (1997). The first to show that agonist reagents to 4-1BB can provide protection against tumours by augmenting the activity of cytotoxic T lymphocytes (CTLs).
Melero, I. et al. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur. J. Immunol. 28, 1116–1121 (1998).
Takahashi, C., Mittler, R. S. & Vella, A. T. 4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 162, 5037–5040 (1999).
Guinn, B. A., DeBenedette, M. A., Watts, T. H. & Berinstein, N. L. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J. Immunol. 162, 5003–5010 (1999).
Halstead, E. S., Mueller, Y. M., Altman, J. D. & Katsikis, P. D. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nature Immunol 3, 536–541 (2002).
Takahashi, C., Mittler, R. S. & Vella, A. T. Differential clonal expansion of CD4 and CD8 T cells in response to 4-1BB ligation: contribution of 4-1BB during inflammatory responses. Immunol. Lett. 76, 183–191 (2001).
Bansal-Pakala, P. & Croft, M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137). J. Immunol. 169, 5005–5009 (2002).
Bertram, E. M., Lau, P. & Watts, T. H. Temporal segregation of 4-1BB versus CD28-mediated co-stimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168, 3777–3785 (2002). This paper establishes the sequential action of 4-1BB late in the T-cell response after CD28.
Del Prete, G. et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J. Exp. Med. 182, 1655–1661 (1995).
Bowen, M. A., Lee, R. K., Miragliotta, G., Nam, S. Y. & Podack, E. R. Structure and expression of murine CD30 and its role in cytokine production. J. Immunol. 156, 442–449 (1996).
Nakamura, T. et al. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-γ. J. Immunol. 158, 2090–2098 (1997).
Amakawa, R. et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell 84, 551–562 (1996).
DeYoung, A. L., Duramad, O. & Winoto, A. The TNF receptor family member CD30 is not essential for negative selection. J. Immunol. 165, 6170–6173 (2000).
Saraiva, M., Smith, P., Fallon, P. G. & Alcami, A. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J. Exp. Med. 196, 829–839 (2002). This paper shows that poxvirus produces a homologue of CD30 that can inhibit inflammation controlled by type 1 cytokines.
Podack, E. R., Strbo, N., Sotosec, V. & Muta, H. CD30-governor of memory T cells? Ann. NY Acad. Sci. 975, 101–113 (2002).
Akiba, H. et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-κB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-κB-inducing kinase. J. Biol. Chem. 273, 13353–13358 (1998).
Aizawa, S. et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J. Biol. Chem. 272, 2042–2045 (1997).
Ansieau, S. et al. Tumor necrosis factor receptor-associated factor (TRAF)-1, TRAF-2, and TRAF-3 interact in vivo with the CD30 cytoplasmic domain; TRAF-2 mediates CD30-induced nuclear factor-κB activation. Proc. Natl Acad. Sci. USA 93, 14053–14058 (1996).
Arch, R. H. & Thompson, C. B. 4-1BB and OX40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor-κB. Mol. Cell. Biol. 18, 558–65 (1998).
Duckett, C. S., Gedrich, R. W., Gilfillan, M. C. & Thompson, C. B. Induction of nuclear factor-κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol. Cell. Biol. 17, 1535–1542 (1997).
Hsu, H. et al. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J. Biol. Chem. 272, 13471–13474 (1997).
Kawamata, S., Hori, T., Imura, A., Takaori-Kondo, A. & Uchiyama, T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-κB activation. J. Biol. Chem. 273, 5808–5814 (1998).
Gravestein, L. A. et al. The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. Eur. J. Immunol. 28, 2208–2216 (1998).
Jang, I. K., Lee, Z. H., Kim, Y. J., Kim, S. H. & Kwon, B. S. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-κB. Biochem. Biophys. Res. Commun. 242, 613–620 (1998).
Saoulli, K. et al. CD28-independent, TRAF2-dependent co-stimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 187, 1849–1862 (1998).
Yamamoto, H., Kishimoto, T. & Minamoto, S. NF-κB activation in CD27 signaling: involvement of TNF receptor-associated factors in its signaling and identification of functional region of CD27. J. Immunol. 161, 4753–4759 (1998).
Marsters, S. A. et al. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J. Biol. Chem. 272, 14029–14032 (1997).
Cannons, J. L., Hoeflich, K. P., Woodgett, J. R. & Watts, T. H. Role of the stress kinase pathway in signaling via the T cell co-stimulatory receptor 4-1BB. J. Immunol. 163, 2990–2998 (1999).
Lee, H. W. et al. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J. Immunol. 169, 4882–4888 (2002).
Laderach, D., Movassagh, M., Johnson, A., Mittler, R. S. & Galy, A. 4-1BB co-stimulation enhances human CD8+ T cell priming by augmenting the proliferation and survival of effector CD8+ T cells. Int. Immunol. 14, 1155–1167 (2002).
Brennan, P. et al. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity 7, 679–689 (1997).
Harhaj, E. W. & Sun, S. C. IκB kinases serve as a target of CD28 signaling. J. Biol. Chem . 273, 25185–25190 (1998).
Su, B. et al. JNK is involved in signal integration during co-stimulation of T lymphocytes. Cell 77, 727–736 (1994).
Prasad, K. V. et al. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl Acad. Sci. USA 91, 2834–2838 (1994).
Kane, L. P., Andres, P. G., Howland, K. C., Abbas, A. K. & Weiss, A. Akt provides the CD28 co-stimulatory signal for upregulation of IL-2 and IFN-γ but not TH2 cytokines. Nature Immunol. 2, 37–44 (2001).
Boise, L. H. et al. CD28 co-stimulation can promote T cell survival by enhancing the expression of Bcl-XL . Immunity 3, 87–98 (1995).
Linton, P. J. et al. Co–stimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and TH2 cytokine secretion in vivo. J. Exp. Med. 197, 875–883 (2003).
Kim, M. Y. et al. CD4+CD3− accessory cells co-stimulate primed CD4 T Cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 18, 643–654 (2003). Together with reference 91, this paper defines populations of antigen-presenting cells (APCs), other than dendritic cells, that can deliver late-acting survival signals from tumour-necrosis factor (TNF)-family ligands.
Blazar, B. R. et al. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J. Immunol . 166, 3174–3183 (2001).
Gramaglia, I., Cooper, D., Miner, K. T., Kwon, B. S. & Croft, M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur. J. Immunol. 30, 392–402 (2000).
Cannons, J. L. et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 167, 1313–1324 (2001).
Akiba, H. et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J. Exp. Med. 191, 375–380 (2000).
Jember, A. G., Zuberi, R., Liu, F. T. & Croft, M. Development of allergic inflammation in a murine model of asthma is dependent on the co-stimulatory receptor OX40. J. Exp. Med . 193, 387–392 (2001).
De Jong, R. et al. The CD27− subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur. J. Immunol. 22, 993–999 (1992).
Salek–Ardakani, S. et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 198, 1–12 (2003) This paper shows that OX40 directly controls the secondary response of memory T cells and, similar to its action in the primary response, regulates the frequency of effector cells that are generated.
Kim, Y. J., Brutkiewicz, R. R. & Broxmeyer, H. E. Role of 4-1BB (CD137) in the functional activation of cord blood CD28–CD8+ T cells. Blood 100, 3253–3260 (2002).
Cannons, J. L., Bertram, E. M. & Watts, T. H. Cutting edge: profound defect in T cell responses in TNF receptor-associated factor 2 dominant negative mice. J. Immunol. 169, 2828–2831 (2002).
Yoshioka, T. et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur. J. Immunol. 30, 2815–2823 (2000).
Kim, Y. J., Kim, S. H., Mantel, P. & Kwon, B. S. Human 4-1BB regulates CD28 co-stimulation to promote TH1 cell responses. Eur. J. Immunol. 28, 881–890 (1998).
Bukczynski, J., Wen, T. & Watts, T. H. Co-stimulation of human CD28− T cells by 4-1BB ligand. Eur. J. Immunol. 33, 446–454 (2003).
Maus, M. V. et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nature Biotechnol. 20, 143–148 (2002).
Tsukada, N. et al. Blockade of CD134 (OX40)–CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood 95, 2434–2439 (2000).
Blazar, B. R. et al. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogenic bone marrow transplant (BMT) recipients. Blood 101, 3741–3748 (2003).
Macian, F. et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 (2002).
Sun, Y. et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J. Immunol. 168, 1457–1465 (2002).
Sun, Y. et al. Co-stimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nature Med. 8, 1405–1413 (2002). Together with reference 109, the authors show that an agonist reagent to 4-1BB can suppress rather than enhance an immune response that is ongoing. This indicates that the nature of any co-stimulation therapy might need to be varied depending on the disease and the stage at which it is targeted.
Zhu, G. et al. Progressive depletion of peripheral B lymphocytes in 4-1BB (CD137) ligand/I-Eα-transgenic mice. J. Immunol. 167, 2671–2676 (2001).
Nakajima, A. et al. Involvement of CD70–CD27 interactions in the induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 109, 188–196 (2000).
Weinberg, A. D. et al. Selective depletion of myelin-reactive T cells with the anti-OX40 antibody ameliorates autoimmune encephalomyelitis. Nature Med. 2, 183–189 (1996). The first to report that targeting OX40 can be therapeutically beneficial in a model of disease.
Weinberg, A. D., Wegmann, K. W., Funatake, C. & Whitham, R. H. Blocking OX40–OX40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 162, 1818–1826 (1999).
Nohara, C. et al. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J. Immunol. 166, 2108–2115 (2001).
Ndhlovu, L. C., Ishii, N., Murata, K., Sato, T. & Sugamura, K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J. Immunol. 167, 2991–2999 (2001).
Arestides, R. S. et al. Co-stimulatory molecule OX40L is critical for both TH1 and TH2 responses in allergic inflammation. Eur. J. Immunol. 32, 2874–2880 (2002).
Higgins, L. M. et al. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40–IgG fusion protein, but not with an OX40 ligand–IgG fusion protein. J. Immunol. 162, 486–493 (1999).
Malmstrom, V. et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J. Immunol. 166, 6972–6981 (2001).
Ekkens, M. J. et al. The role of OX40 ligand interactions in the development of the TH2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J. Immunol. 170, 384–393 (2003).
Zhai, Y. et al. LIGHT, a novel ligand for lymphotoxin β receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J. Clin. Invest. 102, 1142–1151 (1998).
Nieland, J. D., Graus, Y. F., Dortmans, Y. E., Kremers, B. L. & Kruisbeek, A. M. CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response. J. Immunother. 21, 225–236 (1998).
Weinberg, A. D. et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 164, 2160–2169 (2000).
Kjaergaard, J. et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 60, 5514–5521 (2000).
Pan, P., Zang, Y., Weber, K., Meseck, M. & Chen, S. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol. Ther. 6, 528–536 (2002).
Gri, G., Gallo, E., Di Carlo, E., Musiani, P. & Colombo, M. P. OX40 ligand-transduced tumor cell vaccine synergizes with GM-CSF and requires CD40-APC signaling to boost the host T cell antitumor response. J. Immunol. 170, 99–106 (2003).
Strome, S. E. et al. Enhanced therapeutic potential of adoptive immunotherapy by in vitro CD28/4-1BB co-stimulation of tumor-reactive T cells against a poorly immunogenic, major histocompatibility complex class I-negative A9p melanoma. J. Immunother. 23, 430–437 (2000).
Mogi, S. et al. Tumour rejection by gene transfer of 4-1BB ligand into a CD80+ murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunol. 101, 541–547 (2000).
Wilcox, R. A. et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J. Clin. Invest. 109, 651–659 (2002).
Ye, Z. et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nature Med. 8, 343–348 (2002).
Author information
Authors and Affiliations
Glossary
- MIXED-LYMPHOCYTE REACTION
-
(MLR). A proliferative and cytokine response that is brought about when T cells from one donor respond to antigen-presenting cells from a second donor. In most cases, this is an allogeneic reaction that involves the recognition of mismatched peptide–MHC molecules.
- T HELPER 1/T HELPER 2 CELLS
-
(TH1/TH2). Distinct subsets of activated CD4+ T cells. TH1 cells produce interferon-γ, lymphotoxin and tumour-necrosis factor, and support cell-mediated immunity. TH2 cells produce interleukin-4 (IL-4), IL-5, IL-9 and IL-13, and support humoral immunity.
- TYPE 1/2 CYTOTOXIC T CELLS (TC1/TC2).
-
A designation that is used to describe subsets of CD8+ cytotoxic T lymphocytes. TC1 cells typically secrete interferon-γ and tumour-necrosis factor and have marked cytotoxic capacity, whereas TC2 cells secrete interleukin-4 (IL-4), IL-5 and IL-10, and are less effective killers.
- NUCLEAR FACTOR-κB
-
(NF-κB). A family of transcription factors that are important for pro-inflammatory and anti-apoptotic responses. They are activated by the phosphorylation and subsequent ubiquitin-dependent proteolytic degradation of their respective inhibitors, known as inhibitor of NF-κB (IκB). Phosphorylation of IκB occurs through tissue-specific kinases, IκB kinase 1 (IKK1) and IKK2.
- JUN N-TERMINAL KINASE
-
(JNK). A mitogen-activated protein (MAP) kinase that phosphorylates JUN and other components of the AP1 (FOS–JUN) group of transcription factors. This pathway has been implicated in promoting cytokine expression and proliferation, and in some cases might regulate apoptosis.
- PROTEIN KINASE B
-
(PKB). A serine/threonine kinase that is phosphorylated and activated by phosphatidylinositol 3-kinase (PI3K), and can regulate cell division and cell survival through actions on cell-cycle inhibitors and both pro- and anti-apoptotic members of the BCL2 family.
Rights and permissions
About this article
Cite this article
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity?. Nat Rev Immunol 3, 609–620 (2003). https://doi.org/10.1038/nri1148
Issue Date:
DOI: https://doi.org/10.1038/nri1148
This article is cited by
-
Enhancing CAR-T cells: unleashing lasting impact potential with phytohemagglutinin activation in in vivo leukemia model
Cancer Gene Therapy (2024)
-
Synthetic dual co-stimulation increases the potency of HIT and TCR-targeted cell therapies
Nature Cancer (2024)
-
4-1BB immunotherapy: advances and hurdles
Experimental & Molecular Medicine (2024)
-
OX40 in the Pathogenesis of Atopic Dermatitis—A New Therapeutic Target
American Journal of Clinical Dermatology (2024)
-
Microbiota-dependent regulation of costimulatory and coinhibitory pathways via innate immune sensors and implications for immunotherapy
Experimental & Molecular Medicine (2023)