Key Points
-
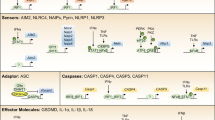
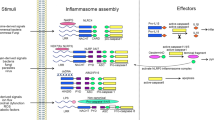
Caspases have multifaceted roles in the immune system. They form structurally diverse and dynamic complexes to drive cell death and inflammation.
-
Inflammatory caspases — caspase 1, caspase 4, caspase 5 and caspase 11 — mediate inflammasome-associated secretion of interleukin-1β (IL-1β) and IL-18 and initiate pyroptosis.
-
The inflammatory caspase 12 has enigmatic roles in the immune system.
-
The apoptotic caspase 8 contributes to the initiation of inflammation by modulating nuclear factor-κB signalling and promotes direct or indirect cleavage of pro-IL-1β and pro-IL-18, but also has a role in the inhibition of inflammation by blocking inflammasome activation, interferon responses and necroptosis.
-
The functional roles of caspases and the cytoskeleton are linked to cell-autonomous immunity, which contributes to the clearance of pathogens.
-
The converging and interconnected roles of caspases challenge the existing functional classification of the caspase family members.
Abstract
Inflammatory and apoptotic caspases are central players in inflammation and apoptosis, respectively. However, recent studies have revealed that these caspases have functions beyond their established roles. In addition to mediating cleavage of the inflammasome-associated cytokines interleukin-1β (IL-1β) and IL-18, inflammatory caspases modulate distinct forms of programmed cell death and coordinate cell-autonomous immunity and other fundamental cellular processes. Certain apoptotic caspases assemble structurally diverse and dynamic complexes that direct inflammasome and interferon responses to fine-tune inflammation. In this Review, we discuss the expanding and interconnected roles of caspases that highlight new aspects of this family of cysteine proteases in innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alnemri, E. S. et al. Human ICE/CED-3 protease nomenclature. Cell 87, 171 (1996).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 (2015).
Stowe, I., Lee, B. & Kayagaki, N. Caspase-11: arming the guards against bacterial infection. Immunol. Rev. 265, 75–84 (2015).
Fuchs, Y. & Steller, H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 16, 329–344 (2015).
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Feoktistova, M. et al. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Tenev, T. et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011).
Brenner, D., Blaser, H. & Mak, T. W. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 15, 362–374 (2015).
Blander, J. M. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat. Rev. Immunol. 14, 601–618 (2014).
Zou, H., Henzel, W. J., Liu, X., Lutschg, A. & Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90, 405–413 (1997).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Srinivasula, S. M., Ahmad, M., Fernandes-Alnemri, T. & Alnemri, E. S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1, 949–957 (1998).
Zou, H., Li, Y., Liu, X. & Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274, 11549–11556 (1999).
Hu, Y., Benedict, M. A., Ding, L. & Nunez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 18, 3586–3595 (1999).
Eckhart, L. et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J. Invest. Dermatol. 115, 1148–1151 (2000).
Lippens, S. et al. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 7, 1218–1224 (2000).
Creagh, E. M. Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol. 35, 631–640 (2014).
Oberst, A. & Green, D. R. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat. Rev. Mol. Cell Biol. 12, 757–763 (2011).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002). This paper describes the existence of an inflammasome complex that is assembled in the cytoplasm to mediate cleavage of IL-1β. This paper also characterizes the basic architecture of the human NLRP1 inflammasome.
Franchi, L., Muñoz-Planillo, R. & Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 (2012).
Kanneganti, T. D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 (2010).
van de Veerdonk, F. L., Joosten, L. A. & Netea, M. G. The interplay between inflammasome activation and antifungal host defense. Immunol. Rev. 265, 172–180 (2015).
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 (2012).
Wen, H., Ting, J. P. & O'Neill, L. A. A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation? Nat. Immunol. 13, 352–357 (2012).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006).
Kang, S. J. et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149, 613–622 (2000).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). This study shows that caspase 11 activation in response to infection by Gram-negative bacteria leads to non-canonical NLRP3 inflammasome activity.
Wang, S. et al. Identification and characterization of Ich-3, a member of the interleukin-1 β converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J. Biol. Chem. 271, 20580–20587 (1996).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon- β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). References 35 and 36 identify an unknown cytoplasmic sensor that mediates recognition of cytosolic LPS independently of TLR4. This sensor was later shown to be caspase 11 (see reference 73).
Casson, C. N. et al. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc. Natl Acad. Sci. USA 112, 6688–6693 (2015).
Schmid-Burgk, J. L. et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 45, 2911–2917 (2015).
Baker, P. J. et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 45, 2918–2926 (2015).
Ruhl, S. & Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K efflux. Eur. J. Immunol. 45, 2927–2936 (2015).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Fassy, F. et al. Enzymatic activity of two caspases related to interleukin-1 β-converting enzyme. Eur. J. Biochem. 253, 76–83 (1998).
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Casson, C. N. et al. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 9, e1003400 (2013).
Lukens, J. R. et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature 498, 224–227 (2013).
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1 β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Sarkar, A. et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am. J. Respir. Crit. Care Med. 174, 1003–1010 (2006).
Kajiwara, Y. et al. A critical role for human caspase-4 in endotoxin sensitivity. J. Immunol. 193, 335–343 (2014).
von Moltke, J. et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111 (2012).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
Jorgensen, I. & Miao, E. A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265, 130–142 (2015).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Sellin, M. E. et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16, 237–248 (2014).
Storek, K. M. & Monack, D. M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 265, 112–129 (2015).
Chen, K. W. et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 (2014).
Mariathasan, S., Weiss, D. S., Dixit, V. M. & Monack, D. M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2014).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). References 62 and 63 demonstrate that the N-terminal fragment of cleaved gasdermin D drives pyroptosis.
Agard, N. J., Maltby, D. & Wells, J. A. Inflammatory stimuli regulate caspase substrate profiles. Mol. Cell Proteom. 9, 880–893 (2010).
Zaki, M. H., Vogel, P., Body-Malapel, M., Lamkanfi, M. & Kanneganti, T. D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 185, 4912–4920 (2010).
Allen, I. C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Dupaul-Chicoine, J. et al. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity 43, 751–763 (2015).
Hu, B. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl Acad. Sci. USA 107, 21635–21640 (2010).
Demon, D. et al. Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis. Mucosal Immunol. 7, 1480–1491 (2014).
Williams, T. M. et al. Caspase-11 attenuates gastrointestinal inflammation and experimental colitis pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G139–G150 (2014).
Oficjalska, K. et al. Protective role for caspase-11 during acute experimental murine colitis. J. Immunol. 194, 1252–1260 (2014). References 69–71independently identify a role for caspase 11 in mediating protection against colitis-associated colorectal cancer.
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors - redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014). This study shows that mouse caspase 11 and human caspase 4 and caspase 5 are sensors of cytosolic LPS. The pathogen-sensing capability of caspases clearly broadens their functional repertoire.
Case, C. L. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl Acad. Sci. USA 110, 1851–1856 (2013).
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014).
Kalai, M. et al. Regulation of the expression and processing of caspase-12. J. Cell Biol. 162, 457–467 (2003).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 (2000).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Fischer, H., Koenig, U., Eckhart, L. & Tschachler, E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 293, 722–726 (2002).
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004).
Xue, Y. et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am. J. Hum. Genet. 78, 659–670 (2006).
Kachapati, K., O'Brien, T. R., Bergeron, J., Zhang, M. & Dean, M. Population distribution of the functional caspase-12 allele. Hum. Mutat. 27, 975 (2006).
Yavari, M. et al. Presence of the functional caspase-12 allele in Indian subpopulations. Int. J. Immunogenet. 39, 389–393 (2012).
Ferwerda, B. et al. Caspase-12 and the inflammatory response to Yersinia pestis. PLoS ONE 4, e6870 (2009).
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006).
Marshall, L. et al. Caspase-12 and rheumatoid arthritis in African-Americans. Immunogenetics 66, 281–285 (2014).
Rosentul, D. C. et al. The impact of caspase-12 on susceptibility to candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 31, 277–280 (2012).
Chen, J. et al. Lack of association of the caspase-12 long allele with community-acquired pneumonia in people of African descent. PLoS ONE 9, e89194 (2014).
McCall, M. B. et al. Persistence of full-length caspase-12 and its relation to malaria in West and Central African populations. Eur. Cytokine Netw. 21, 77–83 (2010).
Hervella, M. et al. The loss of functional caspase-12 in Europe is a pre-neolithic event. PLoS ONE 7, e37022 (2012).
Roy, S. et al. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc. Natl Acad. Sci. USA 105, 4133–4138 (2008).
LeBlanc, P. M. et al. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 3, 146–157 (2008).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Lemmers, B. et al. Essential role for caspase-8 in Toll-like receptors and NF-κB signaling. J. Biol. Chem. 282, 7416–7423 (2007).
Man, S. M. et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol. 191, 5239–5246 (2013).
Gurung, P. et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192, 1835–1846 (2014).
Allam, R. et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990 (2014).
Su, H. et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 307, 1465–1468 (2005). This study identifies a human mutation in caspase 8 that leads to impaired activation of NF-κB signalling, demonstrating a role for caspase 8 in inflammation.
Hu, W. H., Johnson, H. & Shu, H. B. Activation of NF-κB by FADD, Casper, and caspase-8. J. Biol. Chem. 275, 10838–10844 (2000).
Kataoka, T. & Tschopp, J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-κB signaling pathway. Mol. Cell. Biol. 24, 2627–2636 (2004).
Maelfait, J. et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 (2008). This study demonstrates a requirement for TRIF in the processing of IL-1β by caspase 8, but not by caspase 1, in vitro.
Vince, J. E. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Luthi, A. U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Gringhuis, S. I. et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254 (2012). This study characterizes a caspase 8-containing unit induced by certain fungal and mycobacterial infections, leading to direct processing of IL-1β independently of caspase 1.
Moriwaki, K., Bertin, J., Gough, P. J. & Chan, F. K. A. RIPK3-caspase 8 complex mediates atypical pro-IL-1β processing. J. Immunol. 194, 1938–1944 (2015).
Antonopoulos, C., El Sanadi, C., Kaiser, W. J., Mocarski, E. S. & Dubyak, G. R. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. J. Immunol. 191, 4789–4803 (2013).
England, H., Summersgill, H. R., Edye, M. E., Rothwell, N. J. & Brough, D. Release of interleukin-1α or interleukin-1β depends on mechanism of cell death. J. Biol. Chem. 289, 15942–15950 (2014).
Shenderov, K. et al. Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 192, 2029–2033 (2014).
Bossaller, L. et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189, 5508–5512 (2012).
Uchiyama, R., Yonehara, S. & Tsutsui, H. Fas-mediated inflammatory response in Listeria monocytogenes infection. J. Immunol. 190, 4245–4254 (2013).
Man, S. M. et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl Acad. Sci. USA 111, 7403–7408 (2014).
Pierini, R. et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19, 1709–1721 (2012).
Sagulenko, V. et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 20, 1149–1160 (2013).
Antonopoulos, C. et al. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J. Biol. Chem. 290, 20167–20184 (2015).
Weng, D. et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl Acad. Sci. USA 111, 7391–7396 (2014).
Philip, N. H. et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl Acad. Sci. USA 111, 7385–7390 (2014).
Karki, R. et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 17, 357–368 (2015).
Ganesan, S. et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J. Immunol. 193, 2519–2530 (2014).
Lukens, J. R. et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516, 246–249 (2014). This study shows that caspase 1 and caspase 8 operate in a concerted manner to drive production of IL-1β in the development of bone inflammation and destruction.
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Rajput, A. et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity 34, 340–351 (2011).
Kovalenko, A. et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 206, 2161–2177 (2009).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 (2000).
Varfolomeev, E. E. et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Sakamaki, K. et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 9, 1196–1206 (2002).
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Zhang, J., Cado, D., Chen, A., Kabra, N. H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Dillon, C. P. et al. Survival function of the FADD-caspase-8-cFLIP(L) complex. Cell Rep. 1, 401–407 (2012).
Ando, M. et al. Cancer-associated missense mutations of caspase-8 activate nuclear factor-κB signaling. Cancer Sci. 104, 1002–1008 (2013).
Labbe, K., McIntire, C. R., Doiron, K., Leblanc, P. M. & Saleh, M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 35, 897–907 (2011).
Yabal, M. et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 7, 1796–1808 (2014).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Bronner, D. N. et al. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43, 451–462 (2015).
Lebeaupin, C. et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 6, e1879 (2015).
Gurung, P. & Kanneganti, T. D. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am. J. Pathol. 185, 17–25 (2015).
Monie, T. P. & Bryant, C. E. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol. Rev. 265, 181–193 (2015).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004). This paper describes a role for NLRC4 and ASC in the engagement of a caspase 1 inflammasome complex under endogenous settings in response to infection with Salmonella spp.
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006). References 141–143 identify that NLRP3 assembles an inflammasome complex to activate caspase 1 in response to ATP, bacterial RNA molecules and uric acid crystals.
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Broz, P., von Moltke, J., Jones, J. W., Vance, R. E. & Monack, D. M. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Amer, A. et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Zhao, Y. & Shao, F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion. Immunol. Rev. 265, 85–102 (2015).
Yang, J., Zhao, Y., Shi, J. & Shao, F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl Acad. Sci. USA 110, 14408–14413 (2013).
Rayamajhi, M., Zak, D. E., Chavarria-Smith, J., Vance, R. E. & Miao, E. A. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J. Immunol. 191, 3986–3989 (2013).
Kang, S. et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 6, 7515 (2015). References 95–97, 112–118 and 157 are important studies that decipher the functional relevance of caspase 8 in controlling the activities of various caspase 1 inflammasomes.
Ben Moshe, T. et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology 45, 1014–1024 (2007).
Cuda, C. M. et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J. Immunol. 192, 5548–5560 (2014).
Lee, P. et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523 (2009).
Lin, Y., Devin, A., Rodriguez, Y. & Liu, Z. G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 (1999).
Feng, S. et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19, 2056–2067 (2007).
O'Donnell, M. A. et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 13, 1437–1442 (2011).
Gunther, C. et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 (2011).
Weinlich, R. et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 5, 340–348 (2013). References 164 and 165 demonstrate in in vivo models that caspase 8 inhibits inflammation by means of suppressing necroptosis.
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014). References 167 and 168 find that apoptotic caspases of the intrinsic apoptosis pathway negatively regulate cGAS and STING signalling to suppress type I IFN production.
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Kailasan Vanaja, S. et al. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 111, 7765–7770 (2014).
Davidovich, P., Kearney, C. J. & Martin, S. J. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol. Chem. 395, 1163–1171 (2014).
Mostowy, S. & Shenoy, A. R. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat. Rev. Immunol. 15, 559–573 (2015).
Lamkanfi, M. et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell Proteom. 7, 2350–2363 (2008).
Akhter, A. et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361 (2009).
Akhter, A. et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47 (2012).
Li, J. et al. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat. Cell Biol. 9, 276–286 (2007).
Man, S. M. et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl Acad. Sci. USA 111, 17588–17593 (2014).
Kayalar, C., Ord, T., Testa, M. P., Zhong, L. T. & Bredesen, D. E. Cleavage of actin by interleukin 1β-converting enzyme to reverse DNase I inhibition. Proc. Natl Acad. Sci. USA 93, 2234–2238 (1996).
Mashima, T., Naito, M. & Tsuruo, T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene 18, 2423–2430 (1999).
Mashima, T., Naito, M., Fujita, N., Noguchi, K. & Tsuruo, T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem. Biophys. Res. Commun. 217, 1185–1192 (1995).
Puri, A. W., Broz, P., Shen, A., Monack, D. M. & Bogyo, M. Caspase-1 activity is required to bypass macrophage apoptosis upon Salmonella infection. Nat. Chem. Biol. 8, 745–747 (2012).
Kostura, M. J. et al. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc. Natl Acad. Sci. USA 86, 5227–5231 (1989).
Black, R. A., Kronheim, S. R. & Sleath, P. R. Activation of interleukin-1β by a co-induced protease. FEBS Lett. 247, 386–390 (1989).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992).
Ghayur, T. et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386, 619–623 (1997).
Gavrilin, M. A. et al. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J. Immunol. 188, 3469–3477 (2012).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Lin, X. Y., Choi, M. S. & Porter, A. G. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-γ. J. Biol. Chem. 275, 39920–39926 (2000).
Hellmich, K. A. et al. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS ONE 7, e49741 (2012).
Chavarria-Smith, J. & Vance, R. E. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 9, e1003452 (2013).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Man, S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Meunier, E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015).
Acknowledgements
Research studies from the authors' laboratory are supported by the US National Institutes of Health (AR056296, CA163507 and AI101935 to T.-D.K.), the American Lebanese Syrian Associated Charities (to T.-D.K.) and the National Health and Medical Research Council of Australia (NHMRC/R.G. Menzies Early Career Fellowship to S.M.M.). The authors apologize to their colleagues whose work was not cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Inflammasomes
-
Multi-protein complexes that activate caspase 1 to induce processing of pro-interleukin-1β and pro-interleukin-18 and to drive pyroptosis.
- Pyroptosis
-
An inflammatory and lytic form of programmed cell death mediated by inflammatory caspases.
- Apoptosis
-
A non-inflammatory form of programmed cell death mediated by apoptotic caspases. Apoptotic cells exhibit membrane blebbing (but have an intact cell membrane), DNA fragmentation and cell shrinkage.
- Death receptors
-
A subset of receptors within the tumour necrosis factor receptor superfamily that, on binding to their ligands, convey cell death signals.
- FLICE-like inhibitory protein
-
(FLIP). The long isoform (FLIPL) heterodimerizes with pro-caspase 8 to inhibit apoptosis and potentially inhibits necroptosis. By contrast, the short isoform (FLIPS) promotes necroptosis.
- Apoptosome
-
A multi-protein complex comprised of apoptotic protease-activating factor 1 (APAF1) and caspase 9, which induces the intrinsic pathway of apoptosis.
- Microorganism-associated molecular patterns
-
(MAMPs). Conserved structures that constitute microorganisms (for example, lipopolysaccharide or flagellin), which are recognized by pattern-recognition receptors.
- Damage-associated molecular patterns
-
(DAMPs). Molecules that are released by injured cells (for example, ATP or host DNA) and are recognized by pattern-recognition receptors.
- Alarmin
-
A damage-associated molecular pattern that is released by damaged or necrotic cells in response to infection or injury. Alarmins are recognized by pattern-recognition receptors to further activate immune cells.
- Non-canonical NLRP3 inflammasome
-
An inflammasome containing NLRP3 that is activated by Gram-negative bacteria or lipopolysaccharide, requiring activation of caspase 4 or caspase 5 in human cells and caspase 11 in mouse cells.
- Canonical inflammasomes
-
Inflammasomes that do not require caspase 4, caspase 5 and caspase 11 for their activation, including the canonical NLRP3, NLRC4 and AIM2 inflammasomes.
- Guanylate-binding proteins
-
A group of interferon-inducible GTPases produced by the host cell that often target pathogen-containing vacuoles, contributing to the release of pathogens from the vacuole and mediating pathogen killing.
- Necroptosis
-
A type of necrosis and a form of non-apoptotic cell death driven by the kinases RIPK1 and RIPK3 under conditions in which caspase 8 is inhibited.
- Inhibitor of apoptosis proteins
-
(IAPs). A family of proteins that have multiple functions, including inhibition of apoptosis by binding to apoptotic caspases, E3 ubiquitin ligase activity, regulation of MAPK and NF-κB signalling pathways and regulation of signal transduction by pattern-recognition receptors.
- Second mitochondrial-derived activator of caspases
-
(SMAC). Endogenous antagonist of inhibitor of apoptosis proteins (IAPs) released from mitochondria in response to death stimuli. SMAC binds and degrades XIAP, cIAP1 and cIAP2, resulting in increased caspase activation.
- Cell-autonomous immunity
-
A defence mechanism used by the cell to control infection that is not traditionally considered as part of the immune system. Examples include compartmentalization to prevent inappropriate entry of bacteria into the cytoplasm within a eukaryotic cell and production of nitric oxide synthases to mediate killing of an invading microorganism.
Rights and permissions
About this article
Cite this article
Man, S., Kanneganti, TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16, 7–21 (2016). https://doi.org/10.1038/nri.2015.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.7
This article is cited by
-
The relationship between serum resolvin D1, NLRP3, cytokine levels, and adolescents with first-episode medication-naïve major depressive disorder
BMC Psychiatry (2024)
-
Independent role of caspases and Bik in augmenting influenza A virus replication in airway epithelial cells and mice
Virology Journal (2023)
-
Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases
Cell Death & Disease (2023)
-
XCP1 cleaves Pathogenesis-related protein 1 into CAPE9 for systemic immunity in Arabidopsis
Nature Communications (2023)
-
The BCL-2 family protein BCL-RAMBO interacts and cooperates with GRP75 to promote its apoptosis signaling pathway
Scientific Reports (2023)