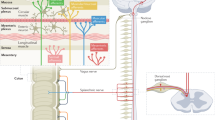
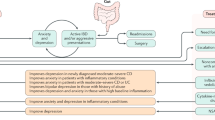
Abstract
The acute phase of IBD with inflamed gut and often ulcerated mucosa is clearly different from the apparently normal mucosa characteristic of IBS. However, more detailed assessment has detected immune activation, increased gut permeability, increased mucosal serotonin availability, abnormalities of enteric nerve structure and function, and dysbiosis in gut microbiota in IBS — all features seen in IBD. Furthermore, as treatments for inflammation in IBD have become more effective it is now apparent that ∼1 in 3 patients with IBD in remission from inflammation still have persistent abnormalities of sensation, motility and gut microbiota, which might cause IBS-like symptoms. This Perspective explores the overlap between IBS and IBD and their treatments, proposing future directions for research in this stimulating area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cooney, R. M., Warren, B. F., Altman, D. G., Abreu, M. T. & Travis, S. P. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials 8, 17 (2007).
Dignass, A. et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J. Crohns Colitis 6, 965–990 (2012).
Longstreth, G. F. et al. Functional bowel disorders. Gastroenterology 130, 1480–1491 (2006).
Shah, E., Rezaie, A., Riddle, M. & Pimentel, M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann. Gastroenterol. 27, 224–230 (2014).
Halpin, S. J. & Ford, A. C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am. J. Gastroenterol. 107, 1474–1482 (2012).
Keohane, J. et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am. J. Gastroenterol. 105, 1789–1794 (2010).
Berrill, J. W., Green, J. T., Hood, K. & Campbell, A. K. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment. Pharmacol. Ther. 38, 44–51 (2013).
Jonefjall, B. et al. Characterization of IBS-like symptoms in patients with ulcerative colitis in clinical remission. Neurogastroenterol. Motil. 25, 756–e578 (2013).
Bernstein, C. N. et al. A prospective population-based study of triggers of symptomatic flares in IBD. Am. J. Gastroenterol. 105, 1994–2002 (2010).
Bernstein, C. N. New insights into IBD epidemiology: are there any lessons for treatment? Dig. Dis. 28, 406–410 (2010).
Cremon, C. et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 104, 392–400 (2009).
Limsui, D. et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm. Bowel Dis. 13, 175–181 (2007).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009).
Gotteland, M. et al. Local and systemic liberation of proinflammatory cytokines in ulcerative colitis. Dig. Dis. Sci. 44, 830–835 (1999).
Dinan, T. G. et al. Hypothalamic–pituitary–gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130, 304–311 (2006).
Liebregts, T. et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 132, 913–920 (2007).
Matricon, J. et al. Review article: associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 36, 1009–1031 (2012).
Vivinus-Nebot, M. et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 63, 744–752 (2014).
Klooker, T. K. et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59, 1213–1221 (2010).
Tibble, J. A., Sigthorsson, G., Foster, R., Forgacs, I. & Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 123, 450–460 (2002).
Langhorst, J. et al. Elevated human β-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am. J. Gastroenterol. 104, 404–410 (2009).
Spiller, R. C. et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment. Pharmacol. Ther. 32, 811–820 (2010).
Koloski, N. A. et al. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 61, 1284–1290 (2012).
Nicholl, B. I. et al. Psychosocial risk markers for new onset irritable bowel syndrome — results of a large prospective population-based study. Pain 137, 147–155 (2008).
Larsson, M. B. et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 142, 463–472 (2012).
Posserud, I. et al. A combined nutrient and lactulose challenge test allows symptom-based clustering of patients with irritable bowel syndrome. Am. J. Gastroenterol. 108, 786–795 (2013).
Yang, J. et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment. Pharmacol. Ther. 39, 302–311 (2014).
Barreau, F., Salvador-Cartier, C., Houdeau, E., Bueno, L. & Fioramonti, J. Long term alterations of colonic nerve mast cell interactions induced by neonatal maternal deprivation in rats. Gut 57, 582–590 (2008).
Vicario, M. et al. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology 37, 65–77 (2012).
Levenstein, S. et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am. J. Gastroenterol. 95, 1213–1220 (2000).
Brydon, L. et al. Psychological stress activates interleukin-1β gene expression in human mononuclear cells. Brain Behav. Immun. 19, 540–546 (2005).
Maes, M. et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a TH1-like response in stress-induced anxiety. Cytokine 10, 313–318 (1998).
Lennon, E. M. et al. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm. Bowel Dis. 19, 712–719 (2013).
Farhadi, A. et al. Heightened responses to stressors in patients with inflammatory bowel disease. Am. J. Gastroenterol. 100, 1796–1804 (2005).
Mawdsley, J. E., Macey, M. G., Feakins, R. M., Langmead, L. & Rampton, D. S. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology 131, 410–419 (2006).
Boye, B. et al. INSPIRE study: Does stress management improve the course of inflammatory bowel disease and disease-specific quality of life in distressed patients with ulcerative colitis or crohn's disease? A randomized controlled trial. Inflamm. Bowel Dis. 17, 1863–1873 (2011).
Ford, A. C., Talley, N. J., Schoenfeld, P. S., Quigley, E. M. & Moayyedi, P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 58, 367–378 (2009).
Longstreth, G. F. et al. Characteristics of patients with irritable bowel syndrome recruited from three sources: implications for clinical trials. Aliment. Pharmacol. Ther. 15, 959–964 (2001).
Thabane, M., Kottachchi, D. T. & Marshall, J. K. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 26, 535–544 (2007).
Chaudhary, N. A. & Truelove, S. C. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q. J. Med. 31, 307–322 (1962).
Gwee, K. A. Irritable bowel syndrome: psychology, biology, and warfare between false dichotomies. Lancet 347, 1267 (1996).
Neal, K. R., Hebden, J. & Spiller, R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 314, 779–782 (1997).
Gwee, K. A. et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 44, 400–406 (1999).
Dunlop, S. P., Jenkins, D. & Spiller, R. C. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am. J. Gastroenterol. 98, 1578–1583 (2003).
Schwille-Kiuntke, J. et al. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol. Motil. 23, e479–e488 (2011).
Neal, K. R., Barker, L. & Spiller, R. C. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut 51, 410–413 (2002).
Spiller, R. C. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811 (2000).
Johansen, K. et al. Intestinal permeability assessed with polyethylene glycols in children with diarrhea due to rotavirus and common bacterial pathogens in a developing community. J. Pediatr. Gastroenterol. Nutr. 9, 307–313 (1989).
Marshall, J. K. et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 20, 1317–1322 (2004).
Dunlop, S. P. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294 (2006).
Vicario, M. et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav. Immun. 24, 1166–1175 (2010).
Vanuytsel, T. et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63, 1293–1299 (2014).
Soderholm, J. D. et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 123, 1099–1108 (2002).
Keita, A. V. et al. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol. Motil. 25, e406–e417 (2013).
Larauche, M. et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G215–G227 (2009).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Buning, C. et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm. Bowel Dis. 18, 1932–1939 (2012).
Soderholm, J. D. et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: an inherited defect in mucosal defence? Gut 44, 96–100 (1999).
Garcia Rodriguez, L. A., Ruigomez, A. & Panes, J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 130, 1588–1594 (2006).
Gradel, K. O. et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 137, 495–501 (2009).
Porter, C. K., Tribble, D. R., Aliaga, P. A., Halvorson, H. A. & Riddle, M. S. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology 135, 781–786 (2008).
Burgmann, T. et al. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis — how much is irritable bowel syndrome? Clin. Gastroenterol. Hepatol. 4, 614–620 (2006).
Canavan, C., Card, T. & West, J. The incidence of other gastroenterological disease following diagnosis of irritable bowel syndrome in the UK: a cohort study. PLoS ONE 9, e106478 (2014).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut — functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Wheatcroft, J. et al. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. 17, 863–870 (2005).
Motomura, Y. et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in TH1 and TH2 dominant environments. Gut 57, 475–481 (2008).
Wang, H. et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut 56, 949–957 (2007).
Dunlop, S. P., Jenkins, D., Neal, K. R. & Spiller, R. C. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 125, 1651–1659 (2003).
Cremon, C. et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 106, 1290–1298 (2011).
Foley, S. et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 140, 1434–1443 (2011).
Faure, C., Patey, N., Gauthier, C., Brooks, E. M. & Mawe, G. M. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139, 249–258 (2010).
Brown, P. M. et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology 141, 507–516 (2011).
Garsed, K. et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63, 1617–1625 (2014).
Andresen, V. et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin. Gastroenterol. Hepatol. 6, 545–555 (2008).
Dunlop, S. P. et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 349–357 (2005).
Atkinson, W., Lockhart, S., Whorwell, P. J., Keevil, B. & Houghton, L. A. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 130, 34–43 (2006).
El-Salhy, M., Gundersen, D., Hatlebakk, J. G. & Hausken, T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig. Dis. Sci. 57, 3154–3159 (2012).
Magro, F. et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig. Dis. Sci. 47, 216–224 (2002).
Massironi, S., Zilli, A., Cavalcoli, F., Conte, D. & Peracchi, M. Chromogranin A and other enteroendocrine markers in inflammatory bowel disease. Neuropeptides 58, 127–134 (2016).
Sciola, V. et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 15, 867–871 (2009).
Zissimopoulos, A. et al. Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand. J. Gastroenterol. 49, 942–949 (2014).
Minderhoud, I. M., Oldenburg, B., Schipper, M. E., Ter Linde, J. J. & Samsom, M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin. Gastroenterol. Hepatol. 5, 714–720 (2007).
Byers, M. R., Suzuki, H. & Maeda, T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc. Res. Tech. 60, 503–515 (2003).
Simpson, J. et al. Prolonged elevation of galanin and tachykinin expression in mucosal and myenteric enteric nerves in trinitrobenzene sulphonic acid colitis. Neurogastroenterol. Motil. 20, 392–406 (2008).
Liebregts, T. et al. Effect of E. coli Nissle 1917 on post-inflammatory visceral sensory function in a rat model. Neurogastroenterol. Motil. 17, 410–414 (2009).
Hughes, P. A. et al. Post-inflammatory colonic afferent sensitization: different subtypes, different pathways, and different time-courses. Gut 58, 1333–1341 (2005).
Belkind-Gerson, J. et al. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm. Bowel Dis. 21, 870–878 (2015).
Belai, A., Boulos, P. B., Robson, T. & Burnstock, G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut 40, 767–774 (1997).
Keranen, U. et al. Changes in substance P-immunoreactive innervation of human colon associated with ulcerative colitis. Dig. Dis. Sci. 40, 2250–2258 (1995).
Watanabe, T., Kubota, Y. & Muto, T. Substance P containing nerve fibers in ulcerative colitis. Int. J. Colorectal Dis. 13, 61–67 (1998).
de Fontgalland, D., Brookes, S. J., Gibbins, I., Sia, T. C. & Wattchow, D. A. The neurochemical changes in the innervation of human colonic mesenteric and submucosal blood vessels in ulcerative colitis and Crohn's disease. Neurogastroenterol. Motil. 26, 731–744 (2014).
Akbar, A. et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 59, 767–774 (2010).
Akbar, A. et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57, 923–929 (2008).
Rajilic-Stojanovic, M. et al. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am. J. Gastroenterol. 110, 278–287 (2015).
Vandeputte, D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016).
Wright, E. K. et al. Recent advances in characterizing the gastrointestinal microbiome in Crohn's disease: a systematic review. Inflamm. Bowel Dis. 21, 1219–1228 (2015).
Jalanka-Tuovinen, J. et al. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 63, 1737–1745 (2014).
Lopez-Siles, M. et al. Mucosa-associated Faecalibacterium prausnitzii and Escherichia coli co-abundance can distinguish irritable bowel syndrome and inflammatory bowel disease phenotypes. Int. J. Med. Microbiol. 304, 464–475 (2014).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Papa, E. et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS ONE 7, e39242 (2012).
Anderson, J. L. et al. Dietary intake of inulin-type fructans in active and inactive Crohn's disease and healthy controls: a case-control study. J. Crohns Colitis 9, 1024–1031 (2015).
Staudacher, H. M. et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 142, 1510–1518 (2012).
Benjamin, J. L. et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut 60, 923–929 (2011).
Czogalla, B. et al. A meta-analysis of immunogenetic Case–Control Association Studies in irritable bowel syndrome. Neurogastroenterol. Motil. 27, 717–727 (2015).
Zucchelli, M. et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut 60, 1671–1677 (2011).
Swan, C. et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut 62, 985–994 (2013).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Burisch, J. & Munkholm, P. The epidemiology of inflammatory bowel disease. Scand. J. Gastroenterol. 50, 942–951 (2015).
Ragnarsson, G. & Bodemar, G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients' description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur. J. Gastroenterol. Hepatol. 10, 415–421 (1998).
Chey, W. L., Jin, H. O., Lee, M. H., Sun, S. W. & Lee, K. Y. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 96, 1499–1506 (2001).
Chang, L. et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut 47, 497–505 (2000).
Mayer, E. A. et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115, 398–409 (2005).
van Hoboken, E. A. et al. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scand. J. Gastroenterol. 46, 981–987 (2011).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Faure, C. & Giguere, L. Functional gastrointestinal disorders and visceral hypersensitivity in children and adolescents suffering from Crohn's disease. Inflamm. Bowel Dis. 14, 1569–1574 (2008).
Rubio, A. et al. Brain responses to uncertainty about upcoming rectal discomfort in quiescent Crohn's disease — a fMRI study. Neurogastroenterol. Motil. http://dx.doi.org/10.1111/nmo.12844, (2016).
Berman, S. M. et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 28, 349–359 (2008).
Hebden, J. M., Blackshaw, P. E., Perkins, A. C., Wilson, C. G. & Spiller, R. C. Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis. Aliment. Pharmacol. Ther. 14, 155–161 (2000).
Lenicek, M. et al. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm. Bowel Dis. 17, 1322–1327 (2011).
Aziz, I. et al. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on Rome III criteria. Clin. Gastroenterol. Hepatol. 13, 1650–1655 (2015).
Ahn, J. Y. et al. Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig. Dis. Sci. 59, 1001–1011 (2014).
Chen, F. et al. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology 123, 2005–2016 (2002).
Hansen, M. B. & Witte, A. B. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. (Oxf.) 193, 311–323 (2008).
Tsukamoto, K. et al. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R64–R69 (2007).
Lordal, M., Wallen, H., Hjemdahl, P., Beck, O. & Hellstrom, P. M. Concentration-dependent stimulation of intestinal phase III of migrating motor complex by circulating serotonin in humans. Clin. Sci. (Lond.) 94, 663–670 (1998).
Kato, S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol. Pharm. Bull. 36, 1406–1409 (2013).
Linden, D. R., Chen, J. X., Gershon, M. D., Sharkey, K. A. & Mawe, G. M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G207–G216 (2003).
Bischoff, S. C. et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G685–G695 (2009).
Kato, S. et al. Dual role of serotonin in the pathogenesis of indomethacin-induced small intestinal ulceration: pro-ulcerogenic action via 5-HT3 receptors and anti-ulcerogenic action via 5-HT4 receptors. Pharmacol. Res. 66, 226–234 (2012).
Matsumoto, K. et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab. Invest. 92, 769–782 (2012).
Mousavizadeh, K., Rahimian, R., Fakhfouri, G., Aslani, F. S. & Ghafourifar, P. Anti-inflammatory effects of 5-HT receptor antagonist, tropisetron on experimental colitis in rats. Eur. J. Clin. Invest. 39, 375–383 (2009).
Lee, C. H. et al. Frozen versus fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 315, 142–149 (2016).
Colman, R. J. & Rubin, D. T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 8, 1569–1581 (2014).
Tack, J. F. et al. Efficacy of ibodutant, a selective antagonist of neurokinin 2 receptors, in irritable bowel syndrome with diarrhoea (IBS-D): the results of a double-blind, randomised, placebo-controlled, parallel-group phase II study (the IRIS-2). Gastroenterology 144, S92–S93 (2013).
Acknowledgements
This Perspetive is a summary of independent research written by the authors who are funded by the National Institute for Health Research Biomedical Research Unit (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
Both authors made equal contributions to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
R.S. has received research funding from Ironwood and Lesaffre and free drug for clinical trial from Falk Pharma and Norgine. He has also acted on Advisory Boards for Allergan, Almirall, Astellas, Danone and Yuhan. G.M. declares no competing interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Spiller, R., Major, G. IBS and IBD — separate entities or on a spectrum?. Nat Rev Gastroenterol Hepatol 13, 613–621 (2016). https://doi.org/10.1038/nrgastro.2016.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.141
This article is cited by
-
Intestinal serotonergic system is modulated by Toll-like receptor 9
Journal of Physiology and Biochemistry (2022)
-
The serotonin receptor 3E variant is a risk factor for female IBS-D
Journal of Molecular Medicine (2022)
-
The physiological and psychological effects of cognitive behavior therapy on patients with inflammatory bowel disease before COVID-19: a systematic review
BMC Gastroenterology (2021)
-
B cell-activating factor (BAFF) in children with inflammatory bowel disease
Pediatric Research (2021)
-
Redox-active nanoparticles for inflammatory bowel disease
Nano Research (2021)