Key Points
-
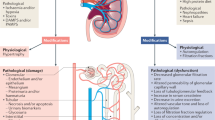
Acute kidney injury (AKI) in cirrhosis consists of a spectrum of diseases, some related to abnormalities in renal function, whereas others are related to renal damage
-
The International Club of Ascites defined AKI in cirrhosis by small changes in serum creatinine and has defined staging, progression and response to treatment
-
Acute or type 1 hepatorenal syndrome (HRS) is now known as HRS-AKI, whereas chronic type 2 HRS is now known as HRS chronic kidney disease
-
The new AKI definition allows patients with persistent doubling of serum creatinine despite albumin and diuretic withdrawal who otherwise fulfil the diagnostic criteria for hepatorenal syndrome to receive pharmacotherapy
-
The use of biomarkers of renal functional changes and structural damage might enhance our ability to assess risk, diagnose AKI and provide a prognosis for patients
-
New knowledge of the pathophysiology of AKI in patients with liver cirrhosis could lead to further refinement of our definition of AKI for cirrhosis in the future
Abstract
Renal dysfunction is prevalent in patients with advanced cirrhosis and decompensation. The presence of type 1 hepatorenal syndrome (HRS) has traditionally been defined by a set of stringent criteria based on serum creatinine levels. These diagnostic criteria have been found to be too stringent to be widely applicable to patients with cirrhosis, leading to underdiagnosis of renal failure in this population. Acute kidney injury (AKI) has now been proposed to characterize renal dysfunction in patients with cirrhosis and is defined as an increase in serum creatinine by 0.3 mg/dl in <48 h or an increase in serum creatinine by 50% from a stable baseline reading within 3 months. Type 1 HRS is renamed HRS-AKI. Stage 1 AKI is defined by 0.3 mg/dl serum creatinine or a 50% increase, stages 2 and 3 AKI are defined by a two-fold and three-fold increase in serum creatinine levels, respectively. Data collected so far suggests that even stage 1 AKI is associated with worse prognosis in patients with cirrhosis. The progression of AKI usually indicates substantially worse outcomes. A panel of biomarkers, including inflammatory markers, are envisaged to complement and enhance our current diagnostic criteria in the future and provide aetiology of the AKI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garcia-Tsao, G., Parikh, C. R. & Viola, A. Acute kidney injury in cirrhosis. Hepatology 48, 2064–2077 (2008).
Salerno, F., Gerbes, A., Ginès, P., Wong, F. & Arroyo, V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 56, 1310–1318 (2007).
Ginès, P., Guevara, M., Arroyo, V. & Rodés, J. Hepatorenal syndrome. Lancet 362, 1819–1827 (2003).
Salerno, F. et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J. Hepatol. 55, 1241–1248 (2011).
Sanyal, A. J. et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 134, 1360–1368 (2008).
Martín-Llahí, M. et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 134, 1352–1359 (2008).
Boyer, T. D. et al. Initial report of a large, randomized, double blind, placebo-controlled, phase 3 trial of terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome (HRS-1): the REVERSE Study. Hepatology 60 (Suppl. 1), 255A (2014).
Boyer, T. D. et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J. Hepatol. 55, 315–321 (2011).
Francoz, C. & Durand, F. Type-1 hepatorenal syndrome in patients with cirrhosis and infection vs. sepsis-induced acute kidney injury: what matters? J. Hepatol. 60, 907–909 (2014).
Rodríguez, E. et al. Terlipressin and albumin for type-1 hepatorenal syndrome associated with sepsis. J. Hepatol. 60, 955–961 (2014).
Adebayo, D., Morabito, V., Davenport, A. & Jalan, R. Renal dysfunction in cirrhosis is not just a vasomotor nephropathy. Kidney Int. 87, 509–515 (2015).
Pipili, C. & Cholongitas, E. Renal dysfunction in patients with cirrhosis: where do we stand? World J. Gastrointest. Pharmacol. Ther. 5, 156–168 (2014).
Macnider, W. D. A study of acute mercuric chloride intoxications in the dog with special reference to the kidney injury. J. Exp. Med. 27, 519–538 (1918).
Bellomo, R., Ronco, C., Kellum, J. A., Mehta, R. L. & Palevsky, P. Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care. 8, R204–R212 (2004).
Hoste, E. A. et al. RIFLE criteria for acute kidney injury is associated with hospital mortality in critical ill patients: a cohort analysis. Crit. Care 10, R73 (2006).
Ali, T. et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J. Am. Soc. Nephrol. 18, 1292–1298 (2007).
Mehta, R. L. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 11, R31 (2007).
Lassnigg, A. et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J. Am. Soc. Nephrol. 15, 1597–1605 (2004).
Joannidis, M. et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 35, 1692–1702 (2009).
International Society of Nephrology. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, S1–S138 (2012).
Jenq, C. C. et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med. 33, 1921–1930 (2007).
Cholongitas, E. et al. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J. Gastroenterol. Hepatol. 24, 1639–1647 (2009).
Rosi, S. et al. New ICA criteria for the diagnosis of acute kidney injury in cirrhotic patients: can we use an imputed value of serum creatinine? Liver Int. 35, 2108–2114 (2015).
Francoz, C., Glotz, D., Moreau, R. & Durand, F. The evaluation of renal function and disease in patients with cirrhosis. J. Hepatol. 52, 605–613 (2010).
Francoz, C. et al. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the Model for End-Stage Liver Disease score. Liver Transpl. 16, 1169–1177 (2010).
Wong, F. Management of ascites in cirrhosis. J. Gastroenterol. Hepatol. 27, 11–20 (2012).
Wong, F. et al. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 60, 702–709 (2011).
Orlando, R. et al. Evaluation of measured and calculated creatinine clearances as glomerular filtration markers in different stages of liver cirrhosis. Clin. Nephrol. 51, 341–347 (1999).
Sherman, D. S., Fish, D. N. & Teitelbaum, I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am. J. Kidney Dis. 41, 269–278 (2003).
Tsien, C. D., Rabie, R. & Wong, F. Acute kidney injury in decompensated cirrhosis. Gut 62, 131–137 (2013).
Tsien, C. & Wong, F. The impact of acute kidney injury in cirrhosis: does definition matter? Gut 62, 1091–1092 (2013).
Wong, F. et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 145, 1280–1289 (2013).
Belcher, J. M. et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 57, 753–762 (2013).
Wong, F. et al. Reduction in acute kidney injury (AKI) stage is a strong predictor of survival in patients with hepatorenal syndrome type-1 (HRS-1) treated with terlipressin plus albumin or albumin alone. Nephrol. Dial. Transplant. 30, Siii451 (2015).
Altamirano, J. et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 10, 65–71 (2012).
Scott, R. A., Austin, A. S., Kolhe, N. V., McIntyre, C. W. & Selby, N. M. Acute kidney injury is independently associated with death in patients with cirrhosis. Frontline Gastroenterol. 4, 191–197 (2013).
Arroyo, V. Acute kidney injury (AKI) in cirrhosis: should we change current definition and diagnostic criteria of renal failure in cirrhosis? J. Hepatol. 59, 415–417 (2013).
Cardenas A. Defining renal failure in cirrhosis—acute kidney injury classification or traditional criteria? Ann. Hepatol. 12, 984–985 (2013).
Sort, P. et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 341, 403–409 (1999).
Angeli, P. et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomized clinical trial. Gut 59, 98–104 (2010).
Piano, S. et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J. Hepatol. 59, 482–489 (2013).
Fagundes, C. et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J. Hepatol. 59, 474–481 (2013).
Wong, F. et al. A cut-off serum creatinine value of 1.5mg/dl for AKI —to be or not to be. J. Hepatol. 62, 741–743 (2015).
Thalhemier, U. & Burroughs, A. K. To close the stable door before the horse has bolted. J. Hepatol. 60, 678–679 (2014).
Piano, S. et al. Reply to: “A cut-off serum creatinine value of 1.5mg/dl for AKI — to be or not to be”. J. Hepatol. 62, 744–746 (2015).
Fagundes, C. et al. Reply to: “A cut-off serum creatinine value of 1.5mg/dl for AKI — to be or not to be”. J. Hepatol. 62, 743–744 (2015).
Wong, F. et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcome. J. Hepatol. 62, S380 (2015).
Angeli, P. et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 64, 531–537 (2015).
McCullough, M. C. et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib. Nephrol. 182, 13–29 (2013).
Murray, P. T. et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 85, 513–521 (2014).
Wong, F. & Murray, P. Kidney damage biomarkers: novel tools for the diagnostic assessment of acute kidney injury in cirrhosis. Hepatology 60, 455–457 (2014).
Parikh, C. R. et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J. Am. Soc. Nephrol. 22, 1748–1757 (2011).
Siew, E. D. et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin. J. Am. Soc. Nephrol. 5, 1497–1505 (2010).
Bolignano, D. et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 52, 595–605 (2008).
Verna, E. C. et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig. Dis. Sci. 57, 2362–2370 (2012).
Fagundes, C. et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J. Hepatol. 57, 267–273 (2012).
Barreto, R. et al. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J. Hepatol. 61, 35–42 (2014).
Ariza, X. et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS ONE 10, e0128145 (2015).
Belcher. J. et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60, 622–632 (2014).
Trawalé, J. M. et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int. 30, 725–732 (2010).
Wong, F. Recent advances in our understanding of hepatorenal syndrome. Nat. Rev. Gastroenterol. Hepatol. 9, 382–391 (2012).
Thabut, D. et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 46, 1872–1882 (2007).
Wong, F., Leung, W., Al Beshir, M., Marquez, M. & Renner, E. L. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 21, 300–307 (2015).
Leithead, J. A. et al. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non-paracetamol-induced acute liver failure. Gut 58, 443–449 (2009).
Wenceslau, C. F., McCarthy, C. G., Szasz, T., Goulopoulou, S. & Webb, R. C. Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am. J. Physiol. Heart Circ. Physiol. 307, H768–H777 (2015).
Gomez, H. et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41, 3–11 (2014).
Garcia-Martinez, R., Noiret, L., Sen, S., Mookerjee, R. & Jalan, R. Albumin infusion improves renal blood flow autoregulation in patients with acute decompensation of cirrhosis and acute kidney injury. Liver Int. 35, 335–343 (2015).
Shah, N. et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J. Hepatol. 56, 1047–1053 (2012).
Shah, N. et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 33, 398–409 (2013).
Arroyo, V. et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 23, 164–176 (1996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Wong, F. The evolving concept of acute kidney injury in patients with cirrhosis. Nat Rev Gastroenterol Hepatol 12, 711–719 (2015). https://doi.org/10.1038/nrgastro.2015.174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.174
This article is cited by
-
Deletion of tumour necrosis factor α receptor 1 elicits an increased TH17 immune response in the chronically inflamed liver
Scientific Reports (2019)
-
Hepatorenal syndrome
Nature Reviews Disease Primers (2018)
-
Acute Kidney Injury in Cirrhosis: Baseline Serum Creatinine Predicts Patient Outcomes
American Journal of Gastroenterology (2017)
-
Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III)
Wiener klinische Wochenschrift (2017)