Abstract
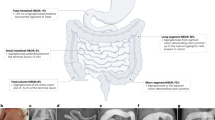
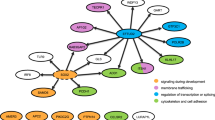
Infantile hypertrophic pyloric stenosis (IHPS) is a common condition in neonates that is characterized by an acquired narrowing of the pylorus. The aetiology of isolated IHPS is still largely unknown. Classic genetic studies have demonstrated an increased risk in families of affected infants. Several genetic studies in groups of individuals with isolated IHPS have identified chromosomal regions linked to the condition; however, these associations could usually not be confirmed in subsequent cohorts, suggesting considerable genetic heterogeneity. IHPS is associated with many clinical syndromes that have known causative mutations. Patients with syndromes associated with IHPS can be considered as having an extreme phenotype of IHPS and studying these patients will be instrumental in finding causes of isolated IHPS. Possible pathways in syndromic IHPS include: (neuro)muscular disorders; connective tissue disorders; metabolic disorders; intracellular signalling pathway disturbances; intercellular communication disturbances; ciliopathies; DNA-repair disturbances; transcription regulation disorders; MAPK-pathway disturbances; lymphatic abnormalities; and environmental factors. Future research should focus on linkage analysis and next-generation molecular techniques in well-defined families with multiple affected members. Studies will have an increased chance of success if detailed phenotyping is applied and if knowledge about the various possible causative pathways is used in evaluating results.
Key Points
-
Genetic factors have an important role in the multifactorial aetiology of infantile hypertrophic pyloric stenosis (IHPS)
-
Family members of affected infants have an increased risk of IHPS
-
Genetic studies have identified different susceptibility loci for IHPS, but these findings could usually not be replicated in subsequent individuals or families, indicating that IHPS has considerable genetic heterogeneity
-
Numerous clinical syndromes are associated with IHPS that result from mutations in genes affecting different pathophysiological pathways
-
Future research should focus on further elucidation of pathophysiological pathways in nonisolated forms of IHPS
-
Next-generation techniques in families and genome-wide association studies in patients with IHPS will probably become successful when detailed phenotyping is applied and if identified pathophysiological pathways are taken into account
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wyllie, R. in Nelson Textbook of Pediatrics (eds Kliegman, R. M., Behrman, R. E., Jenson, H. B. & Stanton, B. F.) 1555–1558 (Saunders Elsevier, Philadelphia, 2007).
Applegate, M. S. & Druschel, C. M. The epidemiology of infantile hypertrophic pyloric stenosis in New York State, 1983 to 1990. Arch. Pediatr. Adolesc. Med. 149, 1123–1129 (1995).
Hedback, G., Abrahamsson, K., Husberg, B., Granholm, T. & Oden, A. The epidemiology of infantile hypertrophic pyloric stenosis in Sweden 1987–96. Arch. Dis. Child. 85, 379–381 (2001).
Nielsen, J. P., Haahr, P. & Haahr, J. Infantile hypertrophic pyloric stenosis. Decreasing incidence. Dan. Med. Bull. 47, 223–225 (2000).
Persson, S., Ekbom, A., Granath, F. & Nordenskjold, A. Parallel incidences of sudden infant death syndrome and infantile hypertrophic pyloric stenosis: a common cause? Pediatrics 108, E70 (2001).
Sommerfield, T. et al. The changing epidemiology of infantile hypertrophic pyloric stenosis in Scotland. Arch. Dis. Child. 93, 1007–1011 (2008).
MacMahon, B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology 17, 195–201 (2006).
Chung, E. et al. Linkage analysis of infantile pyloric stenosis and markers from chromosome 9q11-q33: no evidence for a major gene in this candidate region. J. Med. Genet. 30, 393–395 (1993).
Chung, E. et al. Genetic evidence for the neuronal nitric oxide synthase gene (NOS1) as a susceptibility locus for infantile pyloric stenosis. Am. J. Hum. Genet. 58, 363–370 (1996).
Soderhall, C. & Nordenskjold, A. Neuronal nitric oxide synthase, nNOS, is not linked to infantile hypertrophic pyloric stenosis in three families. Clin. Genet. 53, 421–422 (1998).
Capon, F., Reece, A., Ravindrarajah, R. & Chung, E. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16p12-p13 and evidence for genetic heterogeneity. Am. J. Hum. Genet. 79, 378–382 (2006).
Everett, K. V. et al. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16q24. Eur. J. Hum. Genet. 16, 1151–1154 (2008).
Everett, K. V. et al. Genome-wide high-density SNP-based linkage analysis of infantile hypertrophic pyloric stenosis identifies loci on chromosomes 11q14-q22 and Xq23. Am. J. Hum. Genet. 82, 756–762 (2008).
Svenningsson, A. et al. Genome-wide linkage analysis in families with infantile hypertrophic pyloric stenosis indicates novel susceptibility loci. J. Hum. Genet. 57, 115–121 (2012).
Krogh, C. et al. Testosterone levels in umbilical-cord blood and risk of pyloric stenosis. Pediatrics 127, e197–e201 (2011).
Ranells, J. D., Carver, J. D. & Kirby, R. S. Infantile hypertrophic pyloric stenosis: epidemiology, genetics, and clinical update. Adv. Pediatr. 58, 195–206 (2011).
Sorensen, H. T., Norgard, B., Pedersen, L., Larsen, H. & Johnsen, S. P. Maternal smoking and risk of hypertrophic infantile pyloric stenosis: 10 year population based cohort study. BMJ 325, 1011–1012 (2002).
Dehaene, P. Fetal alcohol syndrome and pyloric stenosis [French]. Arch. Pediatr. 6, 106 (1999).
Lodha, A. K., Satodia, P. & Whyte, H. Fetal alcohol syndrome and pyloric stenosis: alcohol induced or an association? J. Perinat. Med. 33, 262–263 (2005).
Cooper, W. O., Ray, W. A. & Griffin, M. R. Prenatal prescription of macrolide antibiotics and infantile hypertrophic pyloric stenosis. Obstet. Gynecol. 100, 101–106 (2002).
Rogers, I. M. The true cause of pyloric stenosis is hyperacidity. Acta Paediatr. 95, 132–136 (2006).
Honein, M. A. et al. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet 354, 2101–2105 (1999).
Cooper, W. O. et al. Very early exposure to erythromycin and infantile hypertrophic pyloric stenosis. Arch. Pediatr. Adolesc. Med. 156, 647–650 (2002).
Mahon, B. E., Rosenman, M. B. & Kleiman, M. B. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J. Pediatr. 139, 380–384 (2001).
Pedersen, R. N., Garne, E., Loane, M., Korsholm, L. & Husby, S. Infantile hypertrophic pyloric stenosis: a comparative study of incidence and other epidemiological characteristics in seven European regions. J. Matern. Fetal Neonatal Med. 21, 599–604 (2008).
Schechter, R., Torfs, C. P. & Bateson, T. F. The epidemiology of infantile hypertrophic pyloric stenosis. Paediatr. Perinat. Epidemiol. 11, 407–427 (1997).
Rasmussen, L., Green, A. & Hansen, L. P. The epidemiology of infantile hypertrophic pyloric stenosis in a Danish population, 1950–84. Int. J. Epidemiol. 18, 413–417 (1989).
Dahshan, A. et al. Helicobacter pylori and infantile hypertrophic pyloric stenosis: is there a possible relationship? J. Pediatr. Gastroenterol. Nutr. 42, 262–264 (2006).
Sherwood, W., Choudhry, M. & Lakhoo, K. Infantile hypertrophic pyloric stenosis: an infectious cause? Pediatr. Surg. Int. 23, 61–63 (2007).
Burton, P. R., Tobin, M. D. & Hopper, J. L. Key concepts in genetic epidemiology. Lancet 366, 941–951 (2005).
MacMahon, B. & McKeown, T. Infantile hypertrophic pyloric stenosis: data on 81 pairs of twins. Acta Gerontol. (Milano.) 4, 320–329 (1955).
Carter, C. O. & Evans, K. A. Inheritance of congenital pyloric stenosis. J. Med. Genet. 6, 233–254 (1969).
Velaoras, K., Bitsori, M., Galanakis, E. & Charissis, G. Hypertrophic pyloric stenosis in twins: same genes or same environments? Pediatr. Surg. Int. 21, 669–671 (2005).
Carter, C. O. The inheritance of congenital pyloric stenosis. Br. Med. Bull. 17, 251–254 (1961).
Kidd, K. K. & Spence, M. A. Genetic analyses of pyloric stenosis suggesting a specific maternal effect. J. Med. Genet. 13, 290–294 (1976).
Lalouel, J. M., Morton, N. E., MacLean, C. J. & Jackson, J. Recurrence risks in complex inheritance with special regard to pyloric stenosis. J. Med. Genet. 14, 408–414 (1977).
Mitchell, L. E. & Risch, N. The genetics of infantile hypertrophic pyloric stenosis. A reanalysis. Am. J. Dis. Child. 147, 1203–1211 (1993).
Krogh, C. et al. Familial aggregation and heritability of pyloric stenosis. JAMA 303, 2393–2399 (2010).
Yamamoto, Y. et al. Duplication of part of 9q due to maternal 12;9 inverted insertion associated with pyloric stenosis. Am. J. Med. Genet. 31, 379–384 (1988).
Chung, E. et al. Linkage analysis of infantile pyloric stenosis and markers from chromosome 9q11-q33: no evidence for a major gene in this candidate region. J. Med. Genet. 30, 393–395 (1993).
Vanderwinden, J. M., Mailleux, P., Schiffmann, S. N., Vanderhaeghen, J. J. & De Laet, M. H. Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. N. Engl. J. Med. 327, 511–515 (1992).
Takahashi, T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J. Gastroenterol. 38, 421–430 (2003).
Everett, K. V. et al. Infantile hypertrophic pyloric stenosis: evaluation of three positional candidate genes, TRPC1, TRPC5 and TRPC6, by association analysis and re-sequencing. Hum. Genet. 126, 819–831 (2009).
Ju, J. J. et al. No association between the SNPs (rs56134796; rs3824934; rs41302375) in the TRPC6 gene promoter and infantile hypertrophic pyloric stenosis in Chinese people. Pediatr. Surg. Int. 27, 1267–1270 (2011).
Feenstra, B. et al. Common variants near MBNL1 and NKX2–5 are associated with infantile hypertrophic pyloric stenosis. Nat. Genet. 44, 334–337 (2012).
Kusafuka, T. & Puri, P. Altered messenger RNA expression of the neuronal nitric oxide synthase gene in infantile hypertrophic pyloric stenosis. Pediatr. Surg. Int. 12, 576–579 (1997).
Saur, D. et al. Single-nucleotide promoter polymorphism alters transcription of neuronal nitric oxide synthase exon 1c in infantile hypertrophic pyloric stenosis. Proc. Natl Acad. Sci. USA 101, 1662–1667 (2004).
Lagerstedt-Robinson, K., Svenningsson, A. & Nordenskjold, A. No association between a promoter NOS1 polymorphism (rs41279104) and infantile hypertrophic pyloric stenosis. J. Hum. Genet. 54, 706–708 (2009).
Miao, X. et al. Lack of association between nNOS–84G>A polymorphism and risk of infantile hypertrophic pyloric stenosis in a Chinese population. J. Pediatr. Surg. 45, 709–713 (2010).
Serra, A., Schuchardt, K., Genuneit, J., Leriche, C. & Fitze, G. Genomic variants in the coding region of neuronal nitric oxide synthase (NOS1) in infantile hypertrophic pyloric stenosis. J. Pediatr. Surg. 46, 1903–1908 (2011).
Svenningsson, A., Lagerstedt, K., Omrani, M. D. & Nordenskjold, A. Absence of motilin gene mutations in infantile hypertrophic pyloric stenosis. J. Pediatr. Surg. 43, 443–446 (2008).
Serra, A. et al. The role of RET genomic variants in infantile hypertrophic pyloric stenosis. Eur. J. Pediatr. Surg. 21, 389–394 (2011).
Guarino, N., Shima, H. & Puri, P. Structural immaturity of the pylorus muscle in infantile hypertrophic pyloric stenosis. Pediatr. Surg. Int. 16, 282–284 (2000).
Kobayashi, H., O'Briain, D. S. & Puri, P. Immunochemical characterization of neural cell adhesion molecule (NCAM), nitric oxide synthase, and neurofilament protein expression in pyloric muscle of patients with pyloric stenosis. J. Pediatr. Gastroenterol. Nutr. 20, 319–325 (1995).
Piotrowska, A. P., Solari, V. & Puri, P. Distribution of heme oxygenase-2 in nerves and interstitial cells of Cajal in the normal pylorus and in infantile hypertrophic pyloric stenosis. Arch. Pathol. Lab Med. 127, 1182–1186 (2003).
Vanderwinden, J. M., Liu, H., De Laet, M. H. & Vanderhaeghen, J. J. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology 111, 279–288 (1996).
Panteli, C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr. Surg. Int. 25, 1043–1052 (2009).
Dodge, J. A. & Karim, A. A. Induction of pyloric hypertrophy by pentagastrin. An animal model for infantile hypertrophic pyloric stenosis. Gut 17, 280–284 (1976).
Dick, A. C., Ardill, J., Potts, S. R. & Dodge, J. A. Gastrin, somatostatin and infantile hypertrophic pyloric stenosis. Acta Paediatr. 90, 879–882 (2001).
Martinez-Urrutia, M. J., Lassaletta, L., Lama, R., Barrios, V. & Tovar, J. A. Gastric somatostatin content and binding in children with hypertrophic pyloric stenosis: a long-term follow-up study. J. Pediatr. Surg. 30, 1443–1446 (1995).
Huang, P. L., Dawson, T. M., Bredt, D. S., Snyder, S. H. & Fishman, M. C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273–1286 (1993).
Gyurko, R., Leupen, S. & Huang, P. L. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143, 2767–2774 (2002).
Voelker, C. A. et al. Perinatal nitric oxide synthase inhibition retards neonatal growth by inducing hypertrophic pyloric stenosis in rats. Pediatr. Res. 38, 768–774 (1995).
Grisoni, E., Dusleag, D. & Super, D. Nitric oxide synthesis inhibition: the effect on rabbit pyloric muscle. J. Pediatr. Surg. 31, 800–804 (1996).
Barbosa, I. M., Ferrante, S. M. & Mandarim-De-Lacerda, C. A. Role of nitric oxide synthase in the etiopathogenesis of hypertrophic pyloric stenosis in infants [Portuguese]. J. Pediatr. (Rio J.) 77, 307–312 (2001).
Abel, R. M. et al. A histological study of the hph-1 mouse mutant: an animal model of phenylketonuria and infantile hypertrophic pyloric stenosis. Anat. Histol. Embryol. 33, 125–130 (2004).
Johnson, C. F., Koch, R., Peterson, R. M. & Friedman, E. G. Congenital and neurological abnormalities in infants with phenylketonuria. Am. J. Ment. Defic. 82, 375–379 (1978).
Sivarao, D. V., Mashimo, H. & Goyal, R. K. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology 135, 1258–1266 (2008).
Wassif, C. A. et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 10, 555–564 (2001).
Kawauchi, S. et al. Multiple organ system defects and transcriptional dysregulation in the Nipbl+/− mouse, a model of Cornelia de Lange Syndrome. PLoS Genet. 5, e1000650 (2009).
Herman, G. E., Finegold, M., Zhao, W., de Gouyon, B. & Metzenberg, A. Medical complications in long-term survivors with X-linked myotubular myopathy. J. Pediatr. 134, 206–214 (1999).
Robb, S. A. et al. Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul. Disord. 21, 379–386 (2011).
Beighton, P., De Paepe, A., Steinmann, B., Tsipouras, P. & Wenstrup, R. J. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 77, 31–37 (1998).
Zweers, M. C. et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am. J. Hum. Genet. 73, 214–217 (2003).
Narcisi, P., Richards, A. J., Ferguson, S. D. & Pope, F. M. A family with Ehlers-Danlos syndrome type III/articular hypermobility syndrome has a glycine 637 to serine substitution in type III collagen. Hum. Mol. Genet. 3, 1617–1620 (1994).
De Felice, C. et al. Infantile hypertrophic pyloric stenosis and asymptomatic joint hypermobility. J. Pediatr. 138, 596–598 (2001).
Miyazaki, E., Yamataka, T., Ohshiro, K., Taira, Y. & Puri, P. Active collagen synthesis in infantile hypertrophic pyloric stenosis. Pediatr. Surg. Int. 13, 237–239 (1998).
Kelley, R. I. & Hennekam, R. C. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 37, 321–335 (2000).
Tint, G. S. et al. Defective cholesterol biosynthesis associated with the Smith–Lemli–Opitz syndrome. N. Engl. J. Med. 330, 107–113 (1994).
Hennekam, R. C., Waterham, H. R., Wanders, R. J. & Aronson, D. C. No cholesterol metabolism anomalies detectable in infants with hypertrophic pyloric stenosis. Am. J. Med. Genet. 99, 256–257 (2001).
Nezelof, C., Jaubert, F. & Lyon, G. Familial syndrome combining short small intestine, intestinal malrotation, pyloric hypertrophy and brain malformation. 3 anatomoclinical case reports [French]. Ann. Anat. Pathol. (Paris) 21, 401–412 (1976).
Barron, D. J. et al. Hypoplastic left heart syndrome. Lancet 374, 551–564 (2009).
Dasgupta, C. et al. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mutat. Res. 479, 173–186 (2001).
Hardy, C. Pyloromyotomy in an infant with hypoplastic left heart syndrome status-post hybrid procedure: not just another case? Paediatr. Anaesth. 18, 993–994 (2008).
Paznekas, W. A. et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408–418 (2003).
Trarbach, E. B. et al. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J. Clin. Endocrinol. Metab. 95, 3491–3496 (2010).
Franco, B. et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353, 529–536 (1991).
Baker, K. et al. Neocortical and hippocampal volume loss in a human ciliopathy: a quantitative MRI study in Bardet-Biedl syndrome. Am. J. Med. Genet. A 155A, 1–8 (2010).
Weissortel, R., Strom, T. M., Dorr, H. G., Rauch, A. & Meitinger, T. Analysis of an interstitial deletion in a patient with Kallmann syndrome, X-linked ichthyosis and mental retardation. Clin. Genet. 54, 45–51 (1998).
Hennekam, R. C., Krantz, I. D. & Allanson, J. E. in Gorlin's Syndromes of the Head and Neck 428–434 (Oxford University Press, New York, 2010).
Jackson, L., Kline, A. D., Barr, M. A. & Koch, S. de Lange syndrome: a clinical review of 310 individuals. Am. J. Med. Genet. 47, 940–946 (1993).
Krantz, I. D. et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 36, 631–635 (2004).
Oliveira, J. et al. Development of NIPBL locus-specific database using LOVD: from novel mutations to further genotype-phenotype correlations in Cornelia de Lange Syndrome. Hum. Mutat. 31, 1216–1222 (2010).
Bingham, C. & Hattersley, A. T. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1β. Nephrol. Dial. Transplant. 19, 2703–2708 (2004).
Bingham, C. et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am. J. Hum. Genet. 68, 219–224 (2001).
Kaplan, B. S., Gordon, I., Pincott, J. & Barratt, T. M. Familial hypoplastic glomerulocystic kidney disease: a definite entity with dominant inheritance. Am. J. Med. Genet. 34, 569–573 (1989).
Wang, D., Braendstrup, O., Larsen, S., Horn, T. & Strandgaard, S. The expression and activity of renal nitric oxide synthase and circulating nitric oxide in polycystic kidney disease rats. APMIS 112, 358–368 (2004).
Hennekam, R. C. Costello syndrome: an overview. Am. J. Med. Genet. C. Semin. Med. Genet. 117C, 42–48 (2003).
Gripp, K. W. et al. Costello syndrome associated with novel germline HRAS mutations: an attenuated phenotype? Am. J. Med. Genet. A 146A, 683–690 (2008).
Blumenthal, O. Pyloristenosis caused by a neurofibroma in a case of Recklinghausen's disease. Chirurg 21, 313–314 (1950).
Alders, M. et al. Evaluation of clinical manifestiations in patients with severe lymphedema with and without CCBE1 mutations. Eur. J. Med. Genet. (in press).
Alders, M. et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 41, 1272–1274 (2009).
Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 (2009).
Engels, H. et al. Further case of Cantu syndrome: exclusion of cryptic subtelomeric chromosome aberrations. Am. J. Med. Genet. 111, 205–209 (2002).
Scarcella, A., De Lucia, A., Pasquariello, M. B. & Gambardella, P. Early death in two sisters with Hennekam syndrome. Am. J. Med. Genet. 93, 181–183 (2000).
Fox, G. F., Challis, D., O'Brien, K. K., Kelly, E. N. & Ryan, G. Congenital chylothorax in siblings. Acta Paediatr. 87, 1010–1012 (1998).
Mangyanda, M. K. et al. Fetal alcohol syndrome and hypertrophic pyloric stenosis in two brothers [French]. Arch. Pediatr. 5, 695–696 (1998).
Schmickel, R. D. Contiguous gene syndromes: a component of recognizable syndromes. J. Pediatr. 109, 231–241 (1986).
Miller, D. T. et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 86, 749–764 (2010).
Feenstra, B. et al. Common variants near MBNL1 and NKX2–5 are associated with infantile hypertrophic pyloric stenosis. Nat. Genet. 44, 334–337 (2012).
Xu, B. et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat. Genet. 43, 864–868 (2011).
Hennekam, R. C. Care for patients with ultra-rare disorders. Eur. J. Med. Genet. 54, 220–224 (2011).
Kaati, G., Bygren, L. O. & Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur. J. Hum. Genet. 10, 682–688 (2002).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Hennekam, R. C. & Biesecker, L. G. Next generation sequencing demands next generation phenotyping. Hum. Mutat. 33, 884–886 (2012).
Czeizel, A. & Tusnady, G. in Aetiologic studies of isolated common congenital abnormalities in Hungary 107–120 (Akademiai Kiado, Budapest, Hungary, 1984).
Adelstein, P. & Fedrick, J. Pyloric stenosis in the Oxford Record Linkage Study area. J. Med. Genet. 13, 439–448 (1976).
Dodge, J. A. Infantile Hypertrophic Pyloric Stenosis. Thesis, University of Wales (1970).
McKeown, T., MacMahon, B. & Record, R. G. The familial incidence of congenital pyloric stenosis. Ann. Eugen. 16, 260–281 (1951).
Everett, K. V. et al. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16q24. Eur. J. Hum. Genet. 16, 1151–1154 (2008).
Rajab, A., Heathcote, K., Joshi, S., Jeffery, S. & Patton, M. Heterogeneity for congenital generalized lipodystrophy in seventeen patients from Oman. Am. J. Med. Genet. 110, 219–225 (2002).
Gossage, D., Perrin, J. M. & Butler, M. G. A 26-month-old child with Marden-Walker syndrome and pyloric stenosis. Am. J. Med. Genet. 26, 915–919 (1987).
Ay, B., Gercek, A., Dogan, V. I., Kiyan, G. & Gogus, Y. F. Pyloromyotomy in a patient with paramyotonia congenita. Anesth. Analg. 98, 68–69 (2004).
Blank, C. E. Apert's syndrome (a type of acrocephalosyndactyly)—observations on a British series of thirty-nine cases. Ann. Hum. Genet. 24, 151–164 (1960).
Beare, J. M., Dodge, J. A. & Nevin, N. C. Cutis gyratum, acanthosis nigricans and other congenital anomalies. A new syndrome. Br. J. Dermatol. 81, 241–247 (1969).
Mahan, J. D., Mauer, S. M., Sibley, R. K. & Vernier, R. L. Congenital nephrotic syndrome: evolution of medical management and results of renal transplantation. J. Pediatr. 105, 549–557 (1984).
Hu, M. et al. A novel mutation of WT1 exon 9 in a patient with Denys–Drash syndrome and pyloric stenosis. Pediatr. Nephrol. 19, 1160–1163 (2004).
Kuivaniemi, H. et al. Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome. IV. An explanation for exon skipping some mutations and not others. J. Biol. Chem. 265, 12067–12074 (1990).
Wilson, C., Aftimos, S., Pereira, A. & McKay, R. Report of two sibs with Knobloch syndrome (encephalocoele and viteroretinal degeneration) and other anomalies. Am. J. Med. Genet. 78, 286–290 (1998).
Keats, T. E., Smith, T. H. & Sweet, D. E. Craniofacial dysotosis with fibrous metaphyseal deffects. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 124, 271–275 (1975).
Cohen, M. J. J. & Maclean, R. E. in Syndromes with craniosynostosis 309–441 (Oxford University Press, New York, 2000).
Raymond, F. L. et al. Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a Marfanoid habitus. Am. J. Hum. Genet. 80, 982–987 (2007).
Hennekam, R. C., Krantz, I. D. & Allanson, J. E. Gorlin's Syndromes of the Head and Neck (Oxford University Press, New York, 2010).
Lin, D. S. et al. Compound heterozygous mutations in PYCR1 further expand the phenotypic spectrum of De Barsy syndrome. Am. J. Med. Genet. A 155A, 3095–3099 (2011).
Cooper, M. K. et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 33, 508–513 (2003).
Garcia Perez, A. & Crespo, M. X-linked ichthyosis associated with hypertrophic pyloric stenosis in three brothers. Clin. Exp. Dermatol. 6, 159–161 (1981).
Stoll, C., Grosshans, E., Binder, P. & Roth, M. P. Hypertrophic pyloric stenosis associated with X-linked ichthyosis in two brothers. Clin. Exp. Dermatol. 8, 61–64 (1983).
Bruno, L., Bocanegra, O. & Magnelli, N. Recessive X-linked ichthyosis associated with hypertrophic pyloric stenosis: a chance occurrence? Clin. Exp. Dermatol. 28, 74–76 (2003).
Grayer, J. Recognition of Zellweger syndrome in infancy. Adv. Neonatal Care 5, 5–13 (2005).
Powers, J. M. The pathology of peroxisomal disorders with pathogenetic considerations. J. Neuropathol. Exp. Neurol. 54, 710–719 (1995).
Tanner, M. S., Smith, B. & Lloyd, J. K. Functional intestinal obstruction due to deficiency of argyrophil neurones in the myenteric plexus. Familial syndrome presenting with short small bowel, malrotation, and pyloric hypertrophy. Arch. Dis. Child. 51, 837–841 (1976).
Auricchio, A. et al. The locus for a novel syndromic form of neuronal intestinal pseudoobstruction maps to Xq28. Am. J. Hum. Genet. 58, 743–748 (1996).
Goldmuntz, E. et al. CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am. J. Hum. Genet. 70, 776–780 (2002).
Battaglia, A., Chines, C. & Carey, J. C. The FG syndrome: report of a large Italian series. Am. J. Med. Genet. A 140, 2075–2079 (2006).
Rott, H. D., Krieg, P., Rutschle, H. & Kraus, C. Multiple malformations in a male and maternal osteopathia strata with cranial sclerosis (OSCS). Genet. Couns. 14, 281–288 (2003).
Schinzel, A., Illig, R. & Prader, A. The ulnar-mammary syndrome: an autosomal dominant pleiotropic gene. Clin. Genet. 32, 160–168 (1987).
Tanner, M. S., Smith, B. & Lloyd, J. K. Functional intestinal obstruction due to deficiency of argyrophil neurones in the myenteric plexus. Familial syndrome presenting with short small bowel, malrotation, and pyloric hypertrophy. Arch. Dis. Child. 51, 837–841 (1976).
Loh, J. P., Haller, J. O., Kassner, E. G., Aloni, A. & Glassberg, K. Dominantly-inherited polycystic kidneys in infants: association with hypertrophic pyloric stenosis. Pediatr. Radiol. 6, 27–31 (1977).
Balci, S. et al. Familial short rib syndrome, type Beemer, with pyloric stenosis and short intestine, one case diagnosed prenatally. Clin. Genet. 39, 298–303 (1991).
Kaplan, B. S., Fay, J., Shah, V., Dillon, M. J. & Barratt, T. M. Autosomal recessive polycystic kidney disease. Pediatr. Nephrol. 3, 43–49 (1989).
Falk, R. E. & Casas, K. A. Chromosome 2q37 deletion: clinical and molecular aspects. Am. J. Med. Genet. C. Semin. Med. Genet. 145C, 357–371 (2007).
Sherwood, M. C., Pincott, J. R., Goodwin, F. J. & Dillon, M. J. Dominantly inherited glomerulonephritis and an unusual skin disease. Arch. Dis. Child 62, 1278–1280 (1987).
Forrester, S., Kovach, M. J., Reynolds, N. M., Urban, R. & Kimonis, V. Manifestations in four males with and an obligate carrier of the Lenz microphthalmia syndrome. Am. J. Med. Genet. 98, 92–100 (2001).
Hilhorst-Hofstee, Y. et al. Radial aplasia, poikiloderma and auto-immune enterocolitis–new syndrome or severe form of Rothmund-Thomson syndrome? Clin. Dysmorphol. 9, 79–85 (2000).
Konig, R., Fuchs, S., Kern, C. & Langenbeck, U. Simpson-Golabi-Behmel syndrome with severe cardiac arrhythmias. Am. J. Med. Genet. 38, 244–247 (1991).
Johnson, J. P. et al. Costello syndrome: phenotype, natural history, differential diagnosis, and possible cause. J. Pediatr. 133, 441–448 (1998).
Barberia, L. E., Saavedra, O. D. & Maroto, E. M. Etiopathogenic analysis of the caries on three patients with Noonan Syndrome. Med. Oral 8, 136–142 (2003).
Scarcella, A., De, L. A., Pasquariello, M. B. & Gambardella, P. Early death in two sisters with Hennekam syndrome. Am. J. Med. Genet. 93, 181–183 (2000).
Van Balkom, I. D. et al. Lymphedema-lymphangiectasia-mental retardation (Hennekam) syndrome: a review. Am. J. Med. Genet. 112, 412–421 (2002).
Pinter, R., Hogge, W. A. & McPherson, E. Infant with severe penicillamine embryopathy born to a woman with Wilson disease. Am. J. Med. Genet. A 128A, 294–298 (2004).
Alkuraya, F. S. Arthrogryposis, perthes disease, and upward gaze palsy: a novel autosomal recessive syndromic form of arthrogryposis. Am. J. Med. Genet. A 155A, 297–300 (2010).
Crow, Y. J. et al. A newly recognized, likely autosomal recessive syndrome comprising agammaglobulinemia, microcephaly, craniosynostosis, severe dermatitis, and other features. Am. J. Med. Genet. A 140, 1131–1135 (2006).
Suthers, G. K., Earley, A. E. & Huson, S. M. A distinctive syndrome of brachycephaly, deafness, cataracts and mental retardation. Clin. Dysmorphol. 2, 342–345 (1993).
Ayme, S. et al. Fryns syndrome: report on 8 new cases. Clin. Genet. 35, 191–201 (1989).
Harrod, M. J., Keele, D. K. & Howard, J. Sr. A syndrome of craniofacial, digital, and genital anomalies. Birth Defects Orig. Artic. Ser. 13, 111–115 (1977).
Jurenka, S. B. & Van Allen, M. I. Additional case of craniofacial and digital anomalies as reported by Harrod. et al. Am. J. Med. Genet. 61, 168–170 (1996).
Jurenka, S. B. & Evans, J. Kaufman oculocerebrofacial syndrome: case report. Am. J. Med. Genet. 3, 15–19 (1979).
Lowry, R. B. & MacLean, J. R. Syndrome of mental retardation, cleft palate, eventration of diaphragm, congenital heart defect, glaucoma, growth failure and craniosynostosis. Birth Defects Orig. Artic. Ser. 13, 203–228 (1977).
van de Kamp, J. M. et al. Bifurcation of the femur with tibial agenesis and additional anomalies. Am. J. Med. Genet. A 138, 45–50 (2005).
Toriello, H. V. et al. Toriello-Carey syndrome: delineation and review. Am. J. Med. Genet. A 123A, 84–90 (2003).
Rabe, H. et al. Yunis-Varon syndrome: the first case of German origin. Clin. Dysmorphol. 5, 217–222 (1996).
Steinbach, P., Wolf, M. & Schmidt, H. Multiple congenital anomalies/mental retardation (MCA/MR) syndrome due to interstitial deletion 1q. Am. J. Med. Genet. 19, 131–136 (1984).
Webb, A. L. et al. Maternal uniparental disomy for chromosome 2 in association with confined placental mosaicism for trisomy 2 and severe intrauterine growth retardation. Prenat. Diagn. 16, 958–962 (1996).
Tyshchenko, N. et al. 1.6Mb deletion in chromosome band 3q29 associated with eye abnormalities. Eur. J. Med. Genet. 52, 128–130 (2009).
Hodgson, S. V., Berry, A. C. & Dunbar, H. M. Two brothers with an unbalanced 8;17 translocation and infantile pyloric stenosis. Clin. Genet. 48, 328–330 (1995).
Roos, A. et al. Submicroscopic unbalanced translocation resulting in del10p/dup13q detected by subtelomere FISH. Eur. J. Med. Genet. 49, 505–510 (2006).
Shokeir, M. H., Ray, M., Hamerton, J. L., Bauder, F. & O'Brien, H. Deletion of the short arm of chromosome no. 10. J. Med. Genet. 12, 99–103 (1975).
Favier, R. et al. Paris-Trousseau syndrome: clinical, hematological, molecular data of ten new cases. Thromb. Haemost. 90, 893–897 (2003).
Cekada, S. et al. Partial trisomy 13q22−>qter and monosomy 18q21−>qter as a result of familial translocation. Acta Paediatr. 88, 675–678 (1999).
Taylor, A. I. Autosomal trisomy syndromes: a detailed study of 27 cases of Edwards' syndrome and 27 cases of Patau's syndrome. J. Med. Genet. 5, 227–252 (1968).
Chen, C. P. et al. A paternally derived inverted duplication of distal 14q with a terminal 14q deletion. Am. J. Med. Genet. A 139A, 146–150 (2005).
Rasmussen, N., Johnsen, N. J. & Thomsen, J. Inherited congenital bilateral atresia of the external auditory canal, congenital bilateral vertical talus and increased interocular distance. Acta Otolaryngol. 88, 296–302 (1979).
Callier, P. et al. Major feeding difficulties in the first reported case of interstitial 20q11.22-q12 microdeletion and molecular cytogenetic characterization. Am. J. Med. Genet. A 140A, 1859–1863 (2006).
Moore, S. W. Down syndrome and the enteric nervous system. Pediatr. Surg. Int. 24, 873–883 (2008).
Freeman, S. B. et al. Congenital gastrointestinal defects in Down syndrome: a report from the Atlanta and National Down Syndrome Projects. Clin. Genet. 75, 180–184 (2009).
Van der Horst, R., Frankel, J. & Grace, J. Congenital hypertrophic pyloric stenosis in phenotypic female twins with X-XX mosaicism. Arch. Dis. Child. 46, 554–556 (1971).
Author information
Authors and Affiliations
Contributions
B. Peeters researched data for the article. All authors contributed equally to the discussion of the article content, writing the article and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Peeters, B., Benninga, M. & Hennekam, R. Infantile hypertrophic pyloric stenosis—genetics and syndromes. Nat Rev Gastroenterol Hepatol 9, 646–660 (2012). https://doi.org/10.1038/nrgastro.2012.133
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.133
This article is cited by
-
Single-incision versus conventional laparoscopic pyloromyotomy for pediatric hypertrophic pyloric stenosis: a systematic review and meta-analysis
International Journal of Colorectal Disease (2023)
-
Hypertrophic pyloric stenosis masked by kidney failure in a male infant with a contiguous gene deletion syndrome at Xp22.31 involving the steroid sulfatase gene: case report
Italian Journal of Pediatrics (2022)
-
Transumbilical single-site two incision laparoscopic pyloromyotomy for pediatric hypertrophic pyloric stenosis
BMC Surgery (2022)
-
Congenital absence of lingual frenum in a non-syndromic patient: a case report
Journal of Medical Case Reports (2019)
-
Co-occurrence of infantile hypertrophic pyloric stenosis and congenital heart defects: a nationwide cohort study
Pediatric Research (2019)