Abstract
Late diagnosis of pancreatic ductal adenocarcinoma (pancreatic cancer) and the limited response to current treatments results in an exceptionally poor prognosis. Advances in our understanding of the molecular events underpinning pancreatic cancer development and metastasis offer the hope of tangible benefits for patients. In-depth mutational analyses have shed light on the genetic abnormalities in pancreatic cancer, providing potential treatment targets. New biological studies in patients and in mouse models have advanced our knowledge of the timing of metastasis of pancreatic cancer, highlighting new directions for the way in which patients are treated. Furthermore, our increasing understanding of the molecular events in tumorigenesis is leading to the identification of biomarkers that enable us to predict response to treatment. A major drawback, however, is the general lack of an adequate systematic approach to advancing the use of biomarkers in cancer drug development, highlighted in a Cancer Biomarkers Collaborative consensus report. In this Review, we summarize the latest insights into the biology of pancreatic cancer, and their repercussions for treatment. We provide an overview of current treatments and, finally, we discuss novel therapeutic approaches, including the role of biomarkers in therapy for pancreatic cancer.
Key Points
-
Pancreatic cancer is genetically a heterogeneous disease that has a limited response to treatment
-
No accepted way of predicting which patients will respond to treatment currently exists
-
Research is building on advances in our understanding of the molecular pathogenesis of pancreatic cancer to identify more sensitive and robust biomarkers
-
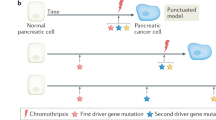
Mouse models and translational studies have further elucidated the timing of metastasis and the therapeutic window in pancreatic cancer
-
The intense stromal response in pancreatic cancer offers one explanation for therapeutic failure and merits further biomarker development
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ferlay, J., Parkin, D. M. & Steliarova-Foucher, E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer 46, 765–781 (2010).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Vincent, A., Herman, J., Schulick, R., Hruban, R. H. & Goggins, M. Pancreatic cancer. Lancet 378, 607–620 (2011).
Lemmens, V. E. et al. Improving outcome for patients with pancreatic cancer through centralization. Br. J. Surg. 98, 1455–1462 (2011).
Ghaneh, P., Costello, E. & Neoptolemos, J. P. Biology and management of pancreatic cancer. Gut 56, 1134–1152 (2007).
Neoptolemos, J. P. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 350, 1200–1210 (2004).
Neoptolemos, J. P. et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br. J. Cancer 100, 246–250 (2009).
Neoptolemos, J. P. et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304, 1073–1081 (2010).
Cunningham, D. et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 27, 5513–5518 (2009).
Stathis, A. & Moore, M. J. Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin. Oncol. 7, 163–172 (2010).
Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008).
Campbell, P. J. et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113 (2010).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Haeno, H. et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 148, 362–375 (2012).
Tuveson, D. A. & Neoptolemos, J. P. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell 148, 21–23 (2012).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 (2011).
Burris, H. A. 3rd et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 (1997).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 (2007).
Dancey, J. E., Bedard, P. L., Onetto, N. & Hudson, T. J. The genetic basis for cancer treatment decisions. Cell 148, 409–420 (2012).
Tisdale, M. J. Cancer cachexia. Langenbecks Arch. Surg. 389, 299–305 (2004).
Felix, K. et al. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 88, 218–225 (2011).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Simon, R. M., Paik, S. & Hayes, D. F. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl Cancer Inst. 101, 1446–1452 (2009).
Erkan, M. et al. The role of stroma in pancreatic cancer—diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. http://dx.doi/org/10.1038/nrgastro.2012.115.
Marechal, R. et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin. Cancer Res. 15, 2913–2919 (2009).
Farrell, J. J. et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 136, 187–195 (2009).
Regine, W. F. et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299, 1019–1026 (2008).
Regine, W. F. et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the, U. S. Intergroup/RTOG 9704 phase III trial. Ann. Surg. Oncol. 18, 1319–1326 (2011).
Oettle, H. et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297, 267–277 (2007).
Costello, E. & Neoptolemos, J. Enhancing the translation of cancer biomarkers. Br. J. Surg. 98, 1039–1040 (2011).
McShane, L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 93, 387–391 (2005).
Khleif, S. N., Doroshow, J. H. & Hait, W. N. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin. Cancer Res. 16, 3299–3318 (2010).
Heinemann, V., Schulz, L., Issels, R. D. & Plunkett, W. Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism. Semin. Oncol. 22, 11–18 (1995).
Marechal, R. et al. Deoxycitidine kinase is associated with prolonged survival after adjuvant gemcitabine for resected pancreatic adenocarcinoma. Cancer 116, 5200–5206 (2010).
Costantino, C. L. et al. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 69, 4567–4572 (2009).
Richards, N. G. et al. HuR status is a powerful marker for prognosis and response to gemcitabine-based chemotherapy for resected pancreatic ductal adenocarcinoma patients. Ann. Surg. 252, 499–505 (2010).
Valsecchi, M. E. et al. Is there a role for the quantification of RRM1 and ERCC1 expression in pancreatic ductal adenocarcinoma? BMC Cancer 12, 104 (2012).
Hummel, R., Hussey, D. J. & Haier, J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur. J. Cancer 46, 298–311 (2010).
Frampton, A. E. et al. microRNAs as markers of survival and chemoresistance in pancreatic ductal adenocarcinoma. Expert Rev. Anticancer Ther. 11, 1837–1842 (2011).
Giovannetti, E. et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 70, 4528–4538 (2010).
Hwang, J. H. et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS ONE 5, e10630 (2010).
Preis, M. et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 17, 5812–5821 (2011).
Bauer, A. S. et al. Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microRNA abundance in blood and tissue. PLoS ONE 7, e34151 (2012).
Miyazono, F. et al. Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am. J. Surg. 177, 475–479 (1999).
de Albuquerque, A. et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology 82, 3–10 (2012).
Kurihara, T. et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J. Hepatobiliary Pancreat. Surg. 15, 189–195 (2008).
Olmos, D. et al. Baseline circulating tumor cell counts significantly enhance a prognostic score for patients participating in phase I oncology trials. Clin. Cancer Res. 17, 5188–5196 (2011).
Neesse, A. et al. Stromal biology and therapy in pancreatic cancer. Gut 60, 861–868 (2010).
Polyak, K., Haviv, I. & Campbell, I. G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 25, 30–38 (2009).
Whiteside, T. L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 27, 5904–5912 (2008).
Hwang, R. F. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 (2008).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Grippo, P. J. & Tuveson, D. A. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev. Res. (Phila.) 3, 1382–1387 (2010).
Infante, J. R. et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 25, 319–325 (2007).
Von Hoff, D. D. et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Frese, K. K. et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2, 261–269 (2012).
Klimstra, D. S. & Longnecker, D. S. K-ras mutations in pancreatic ductal proliferative lesions. Am. J. Pathol. 145, 1547–1550 (1994).
Collins, M. A. et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest. 122, 639–653 (2012).
Grocock, C. J. et al. The variable phenotype of the p.A16V mutation of cationic trypsinogen (PRSS1) in pancreatitis families. Gut 59, 357–363 (2010).
Yan, L. et al. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology 128, 2124–2130 (2005).
Rowinsky, E. K., Windle, J. J. & Von Hoff, D. D. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J. Clin. Oncol. 17, 3631–3652 (1999).
Porzner, M. & Seufferlein, T. Novel approaches to target pancreatic cancer. Curr. Cancer Drug Targets 11, 698–713 (2011).
Bendell, J. & Goldberg, R. M. Targeted agents in the treatment of pancreatic cancer: history and lessons learned. Curr. Opin. Oncol. 19, 390–395 (2007).
da Cunha Santos, G. et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 116, 5599–5607 (2010).
Jimeno, A. et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res. 68, 2841–2849 (2008).
RTOG 0848 protocol information. Radiation Therapy Oncology Group [online], (2012).
Lowery, M. A. & O'Reilly, E. M. New approaches to the treatment of pancreatic cancer: from tumor-directed therapy to immunotherapy. BioDrugs 25, 207–216 (2011).
Middleton, G., Ghaneh, P., Costello, E., Greenhalf, W. & Neoptolemos, J. P. New Treatment options for advanced pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2, 673–696 (2008).
Kindler, H. L. et al. A placebo-controlled, randomized phase II study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G.) in patients (pts) with metastatic pancreatic cancer (mPC) [abstract]. J. Clin. Oncol. a4035 (2010).
Nguyen, L. V., Vanner, R., Dirks, P. & Eaves, C. J. Cancer stem cells: an evolving concept. Nat. Rev. Cancer 12, 133–143 (2012).
McCubrey, J. A. et al. Targeting the cancer initiating cell: the ultimate target for cancer therapy. Curr. Pharm. Des. 18, 1784–1795 (2012).
Shah, A. N. et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 14, 3629–3637 (2007).
Li, C. et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141, 2218–2227 e5 (2011).
Ranganathan, P., Weaver, K. L. & Capobianco, A. J. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer 11, 338–351 (2011).
Ristorcelli, E. & Lombardo, D. Targeting Notch signaling in pancreatic cancer. Expert Opin. Ther. Targets 14, 541–552 (2010).
Wang, Z. et al. Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy. Anticancer Res. 31, 1105–1113 (2011).
Plentz, R. et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 136, 1741–1749 (2009).
Cook, N. et al. Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J. Exp. Med. 209, 437–444 (2012).
Morris, J. P. 4th, Wang, S. C. & Hebrok, M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat. Rev. Cancer 10, 683–695 (2010).
Hidalgo, M. & Maitra, A. The hedgehog pathway and pancreatic cancer. N. Engl. J. Med. 361, 2094–2096 (2009).
Olson, P. & Hanahan, D. Cancer. Breaching the cancer fortress. Science 324, 1400–1401 (2009).
Feldmann, G. et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut 57, 1420–1430 (2008).
Feldmann, G. et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol. Cancer Ther. 7, 2725–2735 (2008).
Stephenson, J. et al. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer [abstract 4114]. J. Clin. Oncol. 29 (2011).
Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer. Infinity Pharmaceuticals [online], (2012).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Hahn, S. A. et al. BRCA2 germline mutations in familial pancreatic carcinoma. J. Natl Cancer Inst. 95, 214–221 (2003).
Bartsch, D. K., Gress, T. M. & Langer, P. Familial pancreatic cancer—current knowledge. Nat. Rev. Gastroenterol. Hepatol. http://dx.doi/org/10.1038/nrgastro.2012.111.
Rouleau, M., Patel, A., Hendzel, M. J., Kaufmann, S. H. & Poirier, G. G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 10, 293–301 (2010).
Fogelman, D. R. et al. Evidence for the efficacy of Iniparib, a PARP-1 inhibitor, in BRCA2-associated pancreatic cancer. Anticancer Res. 31, 1417–1420 (2011).
US National Library of Medicine. Study to assess the safety and tolerability of a PARP inhibitor in combination with gemcitabine in pancreastic cancer. ClinicalTrials.gov [online], (2012).
Sawai, H. et al. The G691S RET polymorphism increases glial cell line-derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res. 65, 11536–11544 (2005).
Ito, Y. et al. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery 138, 788–794 (2005).
Wells, S. A. Jr et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012).
US National Library of Medicine. Clinical trial comparing gemcitabine and vandetanib therapy with gemcitabine alone in pancreatic cancer. ClinicalTrial.gov [online], (2012).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Costello, E., Greenhalf, W. & Neoptolemos, J. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol 9, 435–444 (2012). https://doi.org/10.1038/nrgastro.2012.119
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.119
This article is cited by
-
ENO1 promotes immunosuppression and tumor growth in pancreatic cancer
Clinical and Translational Oncology (2023)
-
C9orf16 represents the aberrant genetic programs and drives the progression of PDAC
BMC Cancer (2022)
-
Pathologische Aufbereitung beim duktalen Adenokarzinom des Pankreas – was gibt es Neues?
Der Chirurg (2022)
-
Blood-based extracellular matrix biomarkers as predictors of survival in patients with metastatic pancreatic ductal adenocarcinoma receiving pegvorhyaluronidase alfa
Journal of Translational Medicine (2021)
-
RNA cargos in extracellular vesicles derived from blood serum in pancreas associated conditions
Scientific Reports (2020)