Abstract
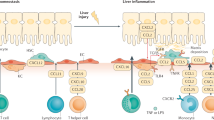
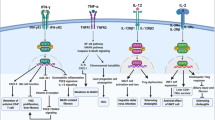
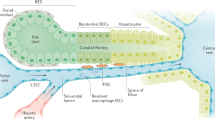
Chemokines are a class of small cytokine-like molecules that orchestrate immune cell infiltration into the liver in response to acute and chronic injuries. Apart from their chemotactic effect, however, chemokines seem to mediate many other aspects of liver diseases, including a direct activation of stellate cells, the modulation of hepatocyte proliferation and angiogenesis. The identification of specific biological functions for chemokines in liver diseases has been hampered by the finding that resident and infiltrating cells in the liver are often a source, as well as a target, of chemokines. Furthermore, chemokines might cause differing effects depending on the etiology of liver damage, their local concentrations and their ability to form multimers and heterodimers. Nevertheless, the functions of a number of important chemokines and their associated receptors have been identified in both in vivo and in vitro studies. Indeed, harmful (proinflammatory, profibrogenic) and beneficial (antifibrogenic, antiangiogenic) effects of chemokines have been discovered in experimental liver disease models. In this Review, the current knowledge of chemokines in experimental liver disease models is summarized. Advances that might lead to preclinical applications are discussed, as are the roles of chemokine receptors as promising pharmacologically targetable molecules.
Key Points
-
Chemokines are soluble mediators that are expressed by most resident and infiltrating cells in the liver following acute and chronic liver injury
-
Chemokines regulate the infiltration of immune cells and stem cells into the liver and modulate the activation and proliferation of liver-resident cells (including hepatocytes, stellate cells and endothelial cells)
-
The effects of chemokines depend on their local concentration and context within the liver as different chemokines are involved in distinct liver diseases at different disease stages
-
Antagonism of single chemokines or their receptors can ameliorate experimental acute and chronic (fibrotic) liver damage in selected models
-
Chemokines are associated with numerous human liver diseases but functional in vivo studies have not been performed in humans to date
-
Chemokine antagonists are currently under clinical investigation for use in human diseases; therefore, further elucidation and characterization of chemokine functions in liver diseases is of great clinical interest
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Luster, A. D. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338, 436–445 (1998).
Bonecchi, R. et al. Chemokines and chemokine receptors: an overview. Front. Biosci. 14, 540–551 (2009).
Colvin, R. A., Campanella, G. S., Sun, J. & Luster, A. D. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J. Biol. Chem. 279, 30219–30227 (2004).
Liu, L., Callahan, M. K., Huang, D. & Ransohoff, R. M. Chemokine receptor CXCR3: an unexpected enigma. Curr. Top. Dev. Biol. 68, 149–181 (2005).
Weber, C. & Koenen, R. R. Fine-tuning leukocyte responses: towards a chemokine 'interactome'. Trends Immunol. 27, 268–273 (2006).
Koenen, R. R. & Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discov. 9, 141–153 (2010).
Wasmuth, H. E., Tacke, F. & Trautwein, C. Chemokines in liver inflammation and fibrosis. Semin. Liver Dis. 30, 215–225 (2010).
Pease, J. E. & Horuk, R. Chemokine receptor antagonists: part 1. Expert Opin. Ther. Pat. 19, 39–58 (2009).
Pease, J. E. & Horuk, R. Chemokine receptor antagonists: part 2. Expert Opin. Ther. Pat. 19, 199–221 (2009).
Heydtmann, M. & Adams, D. H. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology 49, 676–688 (2009).
Lalor, P. F., Faint, J., Aarbodem, Y., Hubscher, S. G. & Adams, D. H. The role of cytokines and chemokines in the development of steatohepatitis. Semin. Liver Dis. 27, 173–193 (2007).
Oo, Y. H., Shetty, S. & Adams, D. H. The role of chemokines in the recruitment of lymphocytes to the liver. Dig. Dis. 28, 31–44 (2010).
Huang, F. & Geng, X. P. Chemokines and hepatocellular carcinoma. World J. Gastroenterol. 16, 1832–1836 (2010).
Tiegs, G., Hentschel, J. & Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Invest. 90, 196–203 (1992).
Zamara, E. et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J. Hepatol. 46, 230–238 (2007).
Berres, M. L. et al. The chemokine scavenging receptor D6 limits acute toxic liver injury in vivo. Biol. Chem. 390, 1039–1045 (2009).
James, L. P., Mayeux, P. R. & Hinson, J. A. Acetaminophen-induced hepatotoxicity. Drug Metab. Dispos. 31, 1499–1506 (2003).
Walsh, K. B., Toledo, A. H., Rivera-Chavez, F. A., Lopez-Neblina, F. & Toledo-Pereyra, L. H. Inflammatory mediators of liver ischemia-reperfusion injury. Exp. Clin. Transplant. 7, 78–93 (2009).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Possamai, L. A. et al. Role of monocytes and macrophages in experimental and human acute liver failure. World J. Gastroenterol. 16, 1811–1819 (2010).
Seki, E. et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 (2009).
Ajuebor, M. N., Hogaboam, C. M., Le, T., Proudfoot, A. E. & Swain, M. G. CCL3/MIP-1α is pro-inflammatory in mouse T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur. J. Immunol. 34, 2907–2918 (2004).
Moreno, C. et al. CCR5 deficiency exacerbates T-cell-mediated hepatitis in mice. Hepatology 42, 854–862 (2005).
Ajuebor, M. N. et al. CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in mouse T cell-mediated hepatitis. Am. J. Pathol. 170, 1975–1988 (2007).
Bernal, W., Auzinger, G., Dhawan, A. & Wendon, J. Acute liver failure. Lancet 376, 190–201 (2010).
Dambach, D. M., Watson, L. M., Gray, K. R., Durham, S. K. & Laskin, D. L. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 35, 1093–1103 (2002).
Si, Y., Tsou, C. L., Croft, K. & Charo, I. F. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J. Clin. Invest. 120, 1192–1203 (2010).
Williams, C. D., Bajt, M. L., Farhood, A. & Jaeschke, H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. doi:10.1111/j.1478-3231.2010.02284.x.
Hu, B. & Colletti, L. M. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology 52, 691–702 (2010).
Bone-Larson, C. L., Hogaboam, C. M., Evanhoff, H., Strieter, R. M. & Kunkel, S. L. IFN-γ-inducible protein-10 (CXCL10) is hepatoprotective during acute liver injury through the induction of CXCR2 on hepatocytes. J. Immunol. 167, 7077–7083 (2001).
Lentsch, A. B., Kato, A., Yoshidome, H., McMasters, K. M. & Edwards, M. J. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 32, 169–173 (2000).
Colletti, L. M. et al. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J. Clin. Invest. 95, 134–141 (1995).
Bertini, R. et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc. Natl Acad. Sci. USA 101, 11791–11796 (2004).
Kuboki, S. et al. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 48, 1213–1223 (2008).
Erhardt, A. & Tiegs, G. Tolerance induction in response to liver inflammation. Dig. Dis. 28, 86–92 (2010).
Eksteen, B. et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J. Immunol. 177, 593–603 (2006).
Graham, G. J. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur. J. Immunol. 39, 342–351 (2009).
Mantovani, A., Bonecchi, R. & Locati, M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat. Rev. Immunol. 6, 907–918 (2006).
Jamieson, T. et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat. Immunol. 6, 403–411 (2005).
Martinez de la Torre, Y. et al. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc. Natl Acad. Sci. USA 104, 2319–2324 (2007).
Wiederholt, T. et al. Genetic variations of the chemokine scavenger receptor D6 are associated with liver inflammation in chronic hepatitis C. Hum. Immunol. 69, 861–866 (2008).
Karlmark, K. R., Wasmuth, H. E., Trautwein, C. & Tacke, F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev. Gastroenterol. Hepatol. 2, 233–242 (2008).
Friedman, S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 (2008).
Holt, A. P. et al. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology 136, 705–714 (2009).
Wasmuth, H. E. et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology 137, 309–319 (2009).
Zaldivar, M. M. et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology 51, 1345–1353 (2010).
Dranoff, J. A. & Wells, R. G. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51, 1438–1444 (2010).
Malik, I. A. et al. Single-dose γ-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am. J. Pathol. 176, 1801–1815 (2010).
Marra, F., Valente, A. J., Pinzani, M. & Abboud, H. E. Cultured human liver fat-storing cells produce monocyte chemotactic protein-1. Regulation by proinflammatory cytokines. J. Clin. Invest. 92, 1674–1680 (1993).
Ramm, G. A. et al. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology 49, 533–544 (2009).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Kruglov, E. A., Nathanson, R. A., Nguyen, T. & Dranoff, J. A. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G765–G771 (2006).
Marra, F. et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am. J. Pathol. 152, 423–430 (1998).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Berres, M. L. et al. Antagonism of the chemokine CCL5 (RANTES) ameliorates experimental liver fibrosis in mice. J. Clin. Invest. (in press).
Schwabe, R. F., Bataller, R. & Brenner, D. A. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G949–G958 (2003).
von Hundelshausen, P., Petersen, F. & Brandt, E. Platelet-derived chemokines in vascular biology. Thromb. Haemost. 97, 704–713 (2007).
von Hundelshausen, P. et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 105, 924–930 (2005).
Koenen, R. R. et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 15, 97–103 (2009).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 11, 1167–1169 (2005).
Lang, P. A. et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat. Med. 14, 756–761 (2008).
Safadi, R. et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology 127, 870–882 (2004).
Goulding, C. et al. The CCR5-Δ32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut 54, 1157–1161 (2005).
Obstfeld, A. E. et al. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925 (2010).
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Marra, F. et al. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology 29, 140–148 (1999).
Mühlbauer, M. et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology 125, 1085–1093 (2003).
Müller, G., Höpken, U. E. & Lipp, M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 195, 117–135 (2003).
Bonacchi, A. et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology 125, 1060–1076 (2003).
Heydtmann, M. et al. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J. Immunol. 177, 729–738 (2006).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 (2007).
Stefanovic, L., Brenner, D. A. & Stefanovic, B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp. Biol. Med. (Maywood) 230, 573–586 (2005).
Hori, Y. et al. Immunohistochemical study of macrophage migration inhibitory factor in rat liver fibrosis induced by thioacetamide. Eur. J. Histochem. 47, 317–324 (2003).
Shi, Z., Wakil, A. E. & Rockey, D. C. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc. Natl Acad. Sci. USA 94, 10663–10668 (1997).
Pesce, J. et al. The IL-21 receptor augments TH2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Syrbe, U., Siveke, J. & Hamann, A. TH1/TH2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin. Immunopathol. 21, 263–285 (1999).
Curbishley, S. M., Eksteen, B., Gladue, R. P., Lalor, P. & Adams, D. H. CXCR3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am. J. Pathol. 167, 887–899 (2005).
Schrage, A. et al. Enhanced T cell transmigration across the mouse liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology 48, 1262–1272 (2008).
Jiang, D. et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J. Clin. Invest. 114, 291–299 (2004).
Nakaya, I. et al. Blockade of IP-10/CXCR3 promotes progressive renal fibrosis. Nephron Exp. Nephrol. 107, e12–e21 (2007).
Santodomingo-Garzon, T., Han, J., Le, T., Yang, Y. & Swain, M. G. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology 49, 1267–1276 (2009).
Oo, Y. H. et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J. Immunol. 184, 2886–2898 (2010).
Tiegs, G. & Lohse, A. W. Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 (2010).
Stockinger, B., Veldhoen, M. & Martin, B. TH17 T cells: linking innate and adaptive immunity. Semin. Immunol. 19, 353–361 (2007).
Rosenblum, J. M. et al. CXCR3 antagonism impairs the development of donor-reactive, IFN-γ-producing effectors and prolongs allograft survival. Transplantation 87, 360–369 (2009).
Barbi, J. et al. Lack of CXCR3 delays the development of hepatic inflammation but does not impair resistance to Leishmania donovani. J. Infect. Dis. 195, 1713–1717 (2007).
Bonacchi, A. et al. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J. Biol. Chem. 276, 9945–9954 (2001).
Strieter, R. M., Burdick, M. D., Gomperts, B. N., Belperio, J. A. & Keane, M. P. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 16, 593–609 (2005).
Fernández, M. et al. Angiogenesis in liver disease. J. Hepatol. 50, 604–620 (2009).
Wasmuth, H. E. et al. The fractalkine receptor CX3CR1 is involved in liver fibrosis due to chronic hepatitis C infection. J. Hepatol. 48, 208–215 (2008).
Efsen, E. et al. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J. Hepatol. 37, 39–47 (2002).
Aoyama, T., Inokuchi, S., Brenner, D. A. & Seki, E. CX3CL1-CX3CR1 interaction prevents CCl4-induced liver inflammation and fibrosis. Hepatology (in press).
Shimoda, S. et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology 51, 567–575 (2010).
Karlmark, K. R. et al. The fractalkine receptor CX3CR1 protects from liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology (in press).
Proudfoot, A. E., Power, C. A. & Schwarz, M. K. Anti-chemokine small molecule drugs: a promising future? Expert Opin. Investig. Drugs 19, 345–355 (2010).
Acknowledgements
Work on chemokines in the laboratory of H. E. Wasmuth is supported by the Deutsche Forschungsgemeinschaft (SFB TRR57 P07/P08) and Aachen University (IZKF grants).
Author information
Authors and Affiliations
Contributions
H. Sahin and H. E. Wasmuth contributed to researching data and writing the article. All authors provided a substantial contribution to discussions of the content and contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sahin, H., Trautwein, C. & Wasmuth, H. Functional role of chemokines in liver disease models. Nat Rev Gastroenterol Hepatol 7, 682–690 (2010). https://doi.org/10.1038/nrgastro.2010.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2010.168
This article is cited by
-
SARS-CoV-2-mediated liver injury: pathophysiology and mechanisms of disease
Inflammation Research (2023)
-
The immunological function of CXCR2 in the liver during sepsis
Journal of Inflammation (2022)
-
Kinin B1 receptor blockade attenuates hepatic fibrosis and portal hypertension in chronic liver diseases in mice
Journal of Translational Medicine (2022)
-
Revealing potential anti-fibrotic mechanism of Ganxianfang formula based on RNA sequence
Chinese Medicine (2022)
-
Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression
Oncogene (2019)