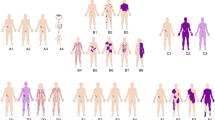
Key Points
-
Mechanisms of mosaicism (the presence of genetically distinct populations of cells in a given organism) include DNA structural abnormalities, epigenetic changes, and the abnormal distribution of chromosomes and mitochondria in daughter cells.
-
Somatic and germ-line mosaicism can coexist in an individual.
-
Several, otherwise lethal, genetic mutations are revealed only in the somatic mosaic state.
-
The Bloom syndrome helicase and the mismatch-repair system are important in the generation of somatic genetic variability.
-
Spontaneous reversions of inherited mutations can occur by homologous recombination, site-specific correction and second-site suppressor mutations.
-
The extent of somatic mosaicism is determined most crucially by selection.
-
Treatment of a genetic disorder can influence the degree of mosaicism and the frequency or maintenance of reverted cell populations.
Abstract
Somatic mosaicism — the presence of genetically distinct populations of somatic cells in a given organism — is frequently masked, but it can also result in major phenotypic changes and reveal the expression of otherwise lethal genetic mutations. Mosaicism can be caused by DNA mutations, epigenetic alterations of DNA, chromosomal abnormalities and the spontaneous reversion of inherited mutations. In this review, we discuss the human disorders that result from somatic mosaicism, as well as the molecular genetic mechanisms by which they arise. Specifically, we emphasize the role of selection in the phenotypic manifestations of mosaicism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gottlieb, B., Beitel, L. K. & Trifiro, M. A. Somatic mosaicism and variable expressivity. Trends Genet. 17, 79–82 (2001).
Wallace, D. C. & Lott, M. in Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 299–409 (Churchill Livingstone, Edinburgh, 2002).
Hall, J. G. Twinning: mechanisms and genetic implications. Curr. Opin. Genet. Dev. 6, 343–347 (1996).
Boveri, T. The Origin of Malignant Tumors (Williams & Wilkins, Baltimore, Maryland, 1929).A timeless classic on somatic mutations and the pathogenesis of cancer.
Jackson, A. L. & Loeb, L. A. The mutation rate and cancer. Genetics 148, 1483–1490 (1998).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Hall, J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am. J. Hum. Genet. 43, 355–363 (1988).An excellent synthesis of observations from clinical genetics that relate to germinal and somatic mosaicism.
Youssoufian, H. Natural gene therapy and the Darwinian legacy. Nature Genet. 13, 255–256 (1996).
Hall, J. G. & Byers, P. H. Genetics of tuberous sclerosis. Lancet 1, 751 (1987).
Van Dijk, B. A., Boomsma, D. I. & De Man, A. J. M. Blood group chimerism in human multiple births is not rare. Am. J. Med. Genet. 61, 264–268 (1996).
Bianchi, D. W. & Lo, Y. M. Fetomaternal cellular and plasma DNA trafficking: the Yin and the Yang. Ann. NY Acad. Sci. 945, 119–131 (2001).
Quaini, F. et al. Chimersim of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Spangrude, G. J., Torok-Storb, B. & Little, M.-T. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 1410–1412 (2002).
Bianchi, D. W., Johnson, K. L. & Salem, D. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 1410–1411 (2002).
Glaser, R., Lu, M. M., Narula, N. & Epstein, J. A. Smooth muscle cells, but not myocytes, of host origin in transplanted hearts. Circulation 106, 17–19 (2002).
Ellis, N. A. et al. Somatic intragenic recombination within the mutated locus BLM can correct the high sister-chromatid exchange phenotype of Bloom syndrome cells. Am. J. Hum. Genet. 57, 1019–1027 (1995).A landmark study on the mechanisms of somatic reversion that facilitated the cloning of the gene that is defective in Bloom syndrome.
Kvittingen, E. A., Rootwelt, H., Berger, R. & Brandtzaeg, P. Self-induced correction of the genetic defect in tyrosinemia type I. J. Clin. Invest. 94, 1657–1661 (1994).
Arredondo-Vega, F. X. et al. Adenosine deaminase deficiency with mosaicism for a 'second-site suppressor' of a splicing mutation: decline in revertant T lymphocytes during enzyme replacement therapy. Blood 99, 1005–1013 (2002).
Gregory, J. J. et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc. Natl Acad. Sci. USA 98, 2532–2537 (2001).
Kazazian, H. H. Retrotransposon insertions in germ cells and somatic cells. Dev. Biol. 106, 307–313 (2001).
Whitelaw, E. & Martin, D. I. K. Retrotransposons as epigenetic mediators of phenotypic variation in mammals. Nature Genet. 27, 361–365 (2001).
Poudrier, J., Lettre, F., Scriver, C. R., Larochelle, J. & Tanguay, R. M. Different clinical forms of hereditary tyrosinemia (type I) in patients with identical genotypes. Mol. Genet. Metab. 64, 119–125 (1998).
Kvittingen, E. A., Rootwelt, H., Brandtzaeg, P., Bergan, A. & Berger, R. Hereditary tyrosinemia type I. Self-induced correction of the fumarylacetoacetase defect. J. Clin. Invest. 91, 1816–1821 (1993).One of the earliest and most complete descriptions of molecular reversion of an inherited mutation.
Yang, T. P. et al. Spontaneous reversion of novel Lesch–Nyhan mutation by HPRT gene rearrangement. Somat. Cell Mol. Genet. 14, 293–303 (1988).
Has, C. et al. The Conradi–Hunermann–Happle syndrome (CDPX2) and emopamil binding protein: novel mutations, and somatic and gonadal mosaicism. Hum. Mol. Genet. 9, 1951–1955 (2000).
Ferguson, H. L. et al. Mosaicism in pseudoachondroplasia. Am. J. Med. Genet. 70, 287–291 (1997).
Montgomery, R. A. et al. Multiple molecular mechanisms underlying subdiagnostic variants of Marfan syndrome. Am. J. Hum. Genet. 63, 1703–1711 (1998).
Burrow, K. L. et al. Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. Neurology 41, 661–668 (1991).
Smith, T. A. et al. Identification and quantification of somatic mosaicism for a point mutation in a Duchenne muscular dystrophy family. J. Med. Genet. 36, 313–315 (1999).
Costa, J.-M. et al. Somatic mosaicism and compound heterozygosity in female hemophilia B. Blood 96, 1585–1587 (2000).
Leuer, M. et al. Somatic mosaicism in hemophilia A: a fairly common event. Am. J. Hum. Genet. 69, 75–87 (2001).
Ainsworth, P. J. et al. Example of somatic mosaicism in a series of de novo neurofibromatosis type 1 cases due to a maternally derived deletion. Hum. Mutat. 9, 452–457 (1997).
Sippel, K. C. et al. Frequency of somatic and germline mosaicism in retinoblastoma: implications for genetic counseling. Am. J. Hum. Genet. 62, 610–619 (1998).
Evans, D. G. R. et al. Somatic mosaicism: a common cause of classic disease in tumor-prone syndromes? Lessons from type 2 neurofibromatosis. Am. J. Hum. Genet. 63, 727–736 (1998).
Verhoef, S. et al. High rate of mosaicism in tuberous sclerosis complex. Am. J. Hum. Genet. 64, 1632–1637 (1999).
Murgia, A. et al. Somatic mosaicism in von Hippel–Lindau disease. Hum. Mutat. 15, 114 (2000).
German, J. & Ellis, N. A. in The Metabolic and Molecular Bases of Inherited Disease 8th Edn (eds Scriver, C., Beaudet, A. L., Sly, W. S. & Valle, D.) 733–752 (McGraw–Hill, New York, 2001).
Ellis, N. A. et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83, 655–666 (1995).
German, J., Ellis, N. A. & Proytcheva, M. Bloom's syndrome. XIX. Cytogenetic and population evidence for genetic heterogeneity. Clin. Genet. 49, 223–231 (1996).
Ellis, N. A., Ciocci, S. & German, J. Back mutation can produce phenotype reversion in Bloom syndrome somatic cells. Hum. Genet. 108, 167–173 (2001).
Auerbach, A. D., Buchwald, M. & Joenje, H. in Genetic Basis of Human Cancer (eds Vogelstein, B. & Kinzler, K. W.) 317–332 (McGraw–Hill, New York, 1998).
Cumming, R. C. et al. Redox regulation of GSTP1 by the Fanconi anemia group C protein prevents apoptosis in hematopoietic cells. Nature Med. 7, 814–820 (2001).
Joenje, H. & Patel, K. J. The emerging genetic and molecular basis of Fanconi anaemia. Nature Rev. Genet. 2, 446–457 (2001).
Kwee, M. L. et al. Unusual response to bifunctional alkylating agents in a case of Fanconi anaemia. Hum. Genet. 64, 384–387 (1983).
Auerbach, A. D. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp. Hematol. 21, 731–733 (1993).
Lo Ten Foe, J. R. et al. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur. J. Hum. Genet. 5, 137–148 (1997).
Waisfisz, Q. et al. Spontaneous functional correction of homozygous Fanconi anaemia alleles reveals novel mechanistic basis for reverse mosaicism. Nature Genet. 22, 379–383 (1999).
Timmers, C. et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol. Cell 7, 241–248 (2001).
Machin, G. A. Some causes of genotypic and phenotypic discordance in monozygotic twin pairs. Am. J. Med. Genet. 61, 216–228 (1996).
Dipple, K. M. & McCabe, R. B. Phenotypes of patients with 'simple' Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am. J. Hum. Genet. 66, 1729–1735 (2000).
Vijg, J. Somatic mutations and aging: a re-evaluation. Mutat. Res. 447, 117–135 (2000).
Bernards, A. & Gusella, J. F. The importance of genetic mosaicism in human disease. N. Engl. J. Med. 331, 1447–1449 (1994).
Cavenee, W. K. et al. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 305, 779–784 (1983).
Lasko, D., Cavenee, W. & Nordenskjold, M. Loss of constitutional heterozygosity in human cancer. Annu. Rev. Genet. 25, 281–314 (1991).
Gupta, P. K. et al. High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination in normal T lymphocytes of human APRT heterozygotes. Cancer Res. 57, 1188–1193 (1997).
De Nooij-Van Dalen, A. G., Morolli, B., van der Marel, A., Lohman, P. H. & Giphart-Gassler, M. Intrinsic genetic instability of normal human lymphocytes and its implication for loss of heterozygosity. Genes Chromosom. Cancer 30, 323–335 (2001).
Tobais, E. S. & Black, D. M. in Principles and Practice of Medical Genetics 4th edn (Eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 514–570 (Churchill Livingstone, Edinburgh, 2002).
Aaltonen, L. A. et al. Clues to the pathogenesis of familial colorectal cancer. Science 260, 812–816 (1993).
Kane, M. F. et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 57, 4749–4756 (1997).
Wong, L.-J. C., Wong, H. & Liu, A. Intergenerational transmission of pathogenic heteroplasmic mitochondrial DNA. Genet. Med. 4, 78–83 (2002).
Orstavik, R. E., Tommerup, N., Eiklid, K. & Orstavik, K. H. Non-random X chromosome inactivation in an affected twin in a monozygotic twin pair discordant for Weidemann–Beckwith syndrome. Am. J. Med. Genet. 56, 210–214 (1995).
Trejo, V., Derom, C., Vlietinck, R. & Ollier, W. X-chromosome inactivation patterns correspond with fetal–placental anatomy in monozygotic twin pairs: implications for immune relatedness and concordance for autoimmunity. Mol. Med. 1, 62–70 (1995).
Bailey, W., Popovich, B. & Jones, K. L. Monozygotic twins discordant for the Russell–Silver syndrome. Am. J. Med. Genet. 58, 101–105 (1995).
Kotzot, D. et al. Uniparental disomy 7 in Silver–Russell syndrome and primordial growth retardation. Hum. Mol. Genet. 4, 583–587 (1995).
Steinmetz, H., Herzog, A., Huang, Y. & Hacklander, T. Discordant brain-surface anatomy in monozygotic twins. N. Engl. J. Med. 331, 952–953 (1994).
Wolf, H. M. et al. Twin boys with major histocompatibility complex class II deficiency but inducible immune responses. N. Engl. J. Med. 332, 86–90 (1995).
Berger, R. & Jonveaux, P. Clonal chromosome abnormalities in Fanconi anemia. Hematol. Cell. Ther. 38, 291–296 (1996).
Alter, B. P. et al. Fanconi anemia: myelodysplasia as a predictor of outcome. Cancer Genet. Cytogenet. 117, 125–131 (2000).
Wallerstein, R. et al. Common trisomy mosaicism diagnosed in amniocytes involving chromosomes 13, 18, 20 and 21: karyotype–phenotype correlations. Prenat. Diagn. 20, 103–122 (2000).
Tomie, J. L. in Principles and Practice of Medical Genetics 4th edn (Eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1129–1182 (Churchill Livingstone, Edinburgh, 2002).
Bamforth, J. S. & Lin, C. C. DK phocomelia phenotype (von Voss–Cherstvoy syndrome) caused by somatic mosaicism for del (13q). Am. J. Med. Genet. 74, 408–411 (1997).An excellent illustration of how phenotypic information that is obtained from clinical genetics can be exploited to identify somatic mosaicism.
Schinzel, A. Tetrasomy 12p (Pallister–Killian syndrome). J. Med. Genet. 28, 122–125 (1991).
Tolmie, J. L. in Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1129–1183 (Churchill Livingstone, Edinburgh, 2002).
Zaragoza, M. et al. Nondisjunction of human acrocentric chromosomes. Studies of 432 fetuses and liveborns. Hum. Genet. 94, 411–417 (1994).
Allanson, J. E. & Graham, G. E. in Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1184–1201 (Churchill Livingstone, Edinburgh, 2002).
Robinson, W. P. Molecular studies of chromosomal mosaicism: relative frequency of chromosome gain or loss and possible role of cell selection. Am. J. Hum. Genet. 56, 444–451 (1995).
Karadima, G. et al. Origins of nondisjunction in trisomy 8 and trisomy 8 mosaicism. Eur. J. Hum. Genet. 6, 432–438 (1998).
Sparkes, R. et al. The validation of a 7-locus multiplex STR test for use in forensic casework. I. Mixtures, ageing, degradation and species studies. Int. J. Legal Med. 109, 186–194 (1996).
Miyamoto, T., Weissman, I. L. & Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl Acad. Sci. USA 97, 7521–7526 (2000).
Arredondo-Vega, F. X. et al. Correct splicing despite mutation of the invariant first nucleotide of a 5′ splice site: a possible basis for disparate clinical phenotypes in siblings with adenosine deaminase deficiency. Am. J. Hum. Genet. 54, 820–830 (1994).
Hirschhorn, R. et al. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nature Genet. 13, 290–295 (1996).
Wada, T. et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc. Natl Acad. Sci. USA 98, 8697–8702 (2001).
Stephan, V. et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N. Engl. J. Med. 335, 1563–1567 (1996).
Joseph, J. T. et al. Congenital myotonic dystrophy pathology and somatic mosaicism. Neurology 49, 1457–1460 (1997).
Van der Maarel, S. M. et al. De novo fascioscapulohumeral muscular dystrophy: frequent somatic mosaicism, sex-dependent phenotype, and the role of mitotic transchromosomal repeat interaction between chromosomes 4 and 10. Am. J. Hum. Genet. 66, 26–35 (2000).
Jonkman, M. F. et al. Revertant mosaicism in epidermolysis bullosa caused by mitotic gene conversion. Cell 88, 543–551 (1997).
Itin, P. H., Buchner, S. A. & Happle, R. Segmental manifestations of Darier disease. What is the genetic background in type 1 and type 2 mosaic phenotypes? Dermatology 200, 254–257 (2000).
Nomura, K., Umeki, K., Hatayama, I. & Kuronuma, T. Phenotypic heterogeneity in bullous congenital ichthyosiform erythroderma: possible somatic mosaicism for keratin gene mutation in the mildly affected mother of the proband. Arch. Dermatol. 137, 1192–1195 (2001).
The International IP Consortium. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter syndrome. Am. J. Hum. Genet. 69, 1210–1217 (2001).This study illustrates the unmasking of lethal genetic mutations in the somatic mosaic state.
Holterhus, P. M. et al. Clinical and molecular spectrum of somatic mosaicism in androgen insensitivity syndrome. Eur. J. Pediatr. 158, 702–706 (1999).
Liehr, T. et al. Mosaicism for the Charcot–Marie–Tooth disease type 1A duplication suggests somatic reversion. Hum. Genet. 98, 22–28 (1996).
Klopstock, T. et al. Markedly different course of Friedreich's ataxia in sib pairs with similar GAA repeat expansions in the frataxin gene. Acta Neuropathol. 97, 139–142 (1999).
Gleeson, G. L. et al. Somatic and germline mosaic mutations in the doublecortin gene are associated with variable phenotypes. Am. J. Hum. Genet. 67, 574–581 (2000).
Armstrong, J., Pineda, M., Aibar, E., Gean, E. & Monros, E. Classic Rett syndrome in a boy as a result of somatic mosaicism for a MECP2 mutation. Ann. Neurol. 50, 692 (2001).
Hashida, H. et al. Single cell analysis of CAG repeat in brains of dentatorubral–pallidoluysian atrophy (DRPLA). J. Neurol. Sci. 190, 87–93 (2001).
Kato, M. et al. Mutation of the doublecortin gene in male patients with double cortex syndrome: somatic mosaicism detected by hair root analysis. Ann. Neurol. 50, 547–551 (2001).
Darling, T. N., Yee, C., Bauer, J. W., Hintner, H. & Yancey, K. B. Revertant mosaicism: partial correction of a germ-line mutation in COL17A1 by a frame-restoring mutation. J. Clin. Invest. 103, 1371–1377 (1999).
Scheuerle, A. E. Male cases of incontinentia pigmenti: case report and review. Am. J. Med. Genet. 77, 201–218 (1998).
Parrish, J. E., Scheuerle, A. E., Lewis, R. A., Levy, M. L. & Nelson, D. L. Selection against mutant alleles in blood leukocytes is a consistent feature in incontinentia pigmenti type 2. Hum. Mol. Genet. 5, 1777–1783 (1996).
Rudolph, D. et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14, 854–862 (2000).
Happle, R. The McCune–Albright syndrome: a lethal gene surviving by mosaicism. Clin. Genet. 29, 321–324 (1986).
Weinstein, L. S. et al. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N. Engl. J. Med. 325, 1688–1695 (1991).
Aldred, M. A. & Trembath, R. C. Activating and inactivating mutations in the human GNAS1 gene. Hum. Mutat. 16, 183–189 (2000).
Bianco, P. et al. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsα-mutated skeletal progenitor cells. J. Clin. Invest. 101, 1737–1744 (1998).
Cohen, M. M. Jr. Proteus syndrome: clinical evidence for somatic mosaicism and selective review. Am. J. Med. Genet. 47, 645–652 (1993).
Biesecker, L. G. The multifaceted challenges of Proteus syndrome. JAMA 285, 2240–2243 (2001).
Stern, C. Somatic crossing over and segregation in Drosophila melanogaster. Genetics 21, 625–730 (1936).
Harrison, B. J. & Carpenter. R. Somatic crossing-over in Antirrhinum majus. Heredity 38, 169–189 (1977).
Lupski, J. R., Roth, J. R. & Weinstock, G. M. Chromosomal duplications in bacteria, fruit flies, and humans. Am. J. Hum. Genet. 58, 21–27 (1996).
Van Sloun, P. P. H. et al. Determination of spontaneous loss of heterozygosity mutation in Aprt heterozygous mice. Nucleic Acids Res. 26, 4888–4894 (1998).
Shao, C. et al. Mitotic recombination produces the majority of the recessive fibroblast variants in heterozygous mice. Proc. Natl Acad. Sci. USA 96, 9230–9235 (1999).
Liang, L., Deng, L., Shao, C., Stambrook, P. J. & Tischfield, J. A. In vivo loss of heterozygosity in T cells of B6C3F1 Aprt+/− mice. Environ. Mol. Mutagen. 35, 150–157 (2000).
Shao, C., Stambrook, P. J. & Tischfield, J. A. Mitotic recombination is suppressed by chromosomal divergence in hybrids of distantly related mouse strains. Nature Genet. 28, 169–172 (2001).An important mechanistic study on somatic mitotic recombination and its implications for tumorigenesis.
Vrieling, H. Mitotic maneuvers in the light. Nature Genet. 28, 101–102 (2001).
Johnson, R. D. & Jasin, M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem. Soc. Trans. 29, 196–201 (2001).
Luo, G. et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 26, 424–429 (2000).
Wu, L. & Hickson, I. D. RecQ helicases and topoisomerases: components of a conserved complex for the regulation of genetic recombination. Cell Mol. Life Sci. 58, 894–901 (2001).
De Wind, N., Dekker, M., Berns, A., Radman, M. & te Riele, H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82, 321–330 (1995).
Shen, P. & Huang, H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112, 441–457 (1986).
Rayssiguier, C., Thaler, D. S. & Radman, M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342, 396–401 (1989).Important mechanistic study of recombination that revealed a role for mismatch repair in prokaryotes.
Datta, A., Hendrix, M., Lipsitch, M. & Jinks-Robertson, S. Dual role for DNA sequence identity and the mismatch repair system in the regulation of mitotic crossing-over in yeast. Proc. Natl Acad. Sci. USA 94, 9757–9762 (1997).
Chen, W. & Jinks-Robertson, S. The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151, 1299–1313 (1999).
Waldman, A. S. & Liskay, R. M. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol. Cell. Biol. 8, 5350–5357 (1988).
Manley, K., Shirley, T. L., Flaherty, L. & Messer, A. MSH2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease and transgenic mice. Nature Genet. 23, 471–473 (1999).
Kovtun, I. V. & McMurray, C. T. Trinucleotide expansion in haploid germ cells by gap repair. Nature Genet. 27, 407–411 (2001).
Battaile, K. P. et al. In vivo selection of wild-type hematopoietic stem cells in a murine model of Fanconi anemia. Blood 94, 2151–2158 (1999).
Haneline, L. S. et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood 94, 1–8 (1999).
Soriano, P. & Jaenisch, R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell 46, 19–29 (1986).
Cohn, D. H., Starman, B. J., Blumberg, B. & Byers, P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am. J. Hum. Genet. 46, 591–601 (1990).An excellent study that combines clinical observation and molecular analysis, and that quantifies the embryonic cellular contribution to the emerging somatic-cell lineages.
Wiemels, J. L. et al. In utero origin of t(8;21) AML1–ETO translocations in childhood acute myeloid leukemia. Blood 99, 3801–3805 (2002).
Flannery, D. B. in Human Malformations and Related Anomalies, Vol. II. Oxford Monographs on Medical Genetics (eds Stevenson, R. E., Hall, J. G. & Goodman, R. M.) 907–930 (Oxford Univ. Press, New York, 1993).
Acknowledgements
We dedicate this review to our mentor and friend, Dr Victor A. McKusick. We would like to thank Andrew Shenker and Robert Tanguny for providing the photos in Figure 1a and 1b, respectively
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
OMIM
adenosine deaminase deficiency
hereditary nonpolyposis colorectal cancer
Glossary
- EXPRESSIVITY
-
The extent to which a particular organ or structure is affected by a particular genotype.
- MCCUNE–ALBRIGHT SYNDROME
-
A human syndrome in which polyostotic lesions affect the skeleton and are patchily distributed. Irregular café au lait hyperpigmented macules affect the skin. Several endocrinopathies are typically present, causing variable hypercortisolism, hyperthyroidism, pituitary gigantism and sexual precocity in girls.
- HEREDITARY TYROSINAEMIA TYPE I
-
An autosomal-recessive disorder of tyrosine metabolism that is due to deficiency of the enzyme fumarylacetoacetate hydrolase. The disorder typically causes liver and renal problems early in life and is associated with neurological deterioration and hepatoma.
- ALLOGENEIC ORGAN TRANSPLANTATION
-
The use of an organ or tissue from any human other than self or a monozygotic twin.
- SEGMENTAL NEUROFIBROMATOSIS TYPE I
-
A form of neurofibromatosis type I in which pigmented skin macules, subcutaneous benign tumors (neurofibroma) or large plexiform neurofibroma are limited to one region of the body. Segmental neurofibromatosis type II also occurs, typically with unilateral vestibular schwannoma or meningioma.
- TUBEROUS SCLEROSIS
-
An autosomal-dominant disorder with a varied phenotype. Severely affected individuals have seizures, mental retardation and fatty tumours of the kidneys. Mildly affected individuals might only show small, depigmented skin macules and small nodules under the nails and on the face.
- ASCERTAINMENT BIAS
-
Any tendency for an inference to be non-representative of the true population, because individuals of a particular class are more likely to be sampled.
- COMPOUND HETEROZYGOTE
-
The occurrence of mutations in each of the two alleles of an autosomal gene.
- GENE CONVERSION
-
A non-reciprocal recombination process that results in an alteration of the sequence of a gene to that of its homologue during meiosis.
- COMPLEMENTATION GROUP C
-
Subclassification of cells from Fanconi anaemia patients on the basis of somatic-cell hybrid analysis or mutation analysis.
- CHORIONIC VILLUS SAMPLING
-
A prenatal diagnostic procedure that involves removal of cells from the finger-like projections (villi) of the chorion (the tissue surrounding the fetus) to obtain chromosomes and cell products for diagnosis.
- AMNIOCENTESIS
-
Prenatal diagnosis method in which cells of the amniotic fluid are used to determine the number and kind of chromosomes of the fetus and for biochemical studies.
- DK-PHOCOMELIA
-
A rare condition that is characterized by maldevelopment of the proximal portions of limbs, urogenital defects and failure of closure of the posterior skull (occipital encephalocoele).
- PALLISTER–KILLIAN SYNDROME
-
A severe congenital malformation syndrome that includes mental retardation, postnatal growth deficiency, progressive coarsening of the facial features, and sparse eyebrows and hair over the temples. It is due to tetrasomy of the short arm of human chromosome 12.
- ISODISOMY
-
A euploid cell in which one of the chromosome pairs consists of identical chromosomes inherited from one parent; this is due to non-disjunction in meiosis II in one parent, formation of a triploid zygote and loss of the chromosome provided by the parent in which non-disjunction did not occur.
- MULTIPLEX PCR
-
Simultaneous analysis of multiple genetic loci by PCR.
- PROTEUS SYNDROME
-
A congenital malformation disorder in which overgrowth of any organ or tissue can occur, but invariably in a patchy distribution.
Rights and permissions
About this article
Cite this article
Youssoufian, H., Pyeritz, R. Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet 3, 748–758 (2002). https://doi.org/10.1038/nrg906
Issue Date:
DOI: https://doi.org/10.1038/nrg906
This article is cited by
-
Investigating the potential roles of intra-colonial genetic variability in Pocillopora corals using genomics
Scientific Reports (2024)
-
Maternal mosaicism in SSBP1 causing optic atrophy with retinal degeneration: implications for genetic counseling
Orphanet Journal of Rare Diseases (2023)
-
Characteristics and mechanisms of mosaicism in prenatal diagnosis cases by application of SNP array
Molecular Cytogenetics (2023)
-
Lymphoid clonal hematopoiesis: implications for malignancy, immunity, and treatment
Blood Cancer Journal (2023)
-
CRELD1 variants are associated with bicuspid aortic valve in Turner syndrome
Human Genetics (2023)