Key Points
-
Although Arabidopsis thaliana has traditionally been the primary model organism in plants, several closely related species have recently become a focal point for comparative genomic studies and have led to an expansion of the spectrum of traits under study.
-
The availability and analysis of several high-quality whole-genome sequences from species closely related to A. thaliana within the Brassicaceae family have shed light on the processes of genome evolution in plants, including the emergence and evolution of polyploids.
-
Comparative genomic studies across species of the Brassicaceae family have identified an important role for local gene duplications and deletions in generating evolutionary diversity and diversification of gene function in plants.
-
Several species of the Brassicaceae family are better subjects for field studies than A. thaliana itself. The use of these species as models has enabled researchers to identify the genetic basis for fitness in the real field conditions.
-
The accelerated production of high-quality whole-genome sequences from a range of plant species, and the emergence of genome-editing technologies, will continue to facilitate advances in the study of non-model species in the Brassicaceae family.
Abstract
For decades a small number of model species have rightly occupied a privileged position in laboratory experiments, but it is becoming increasingly clear that our knowledge of biology is greatly improved when informed by a broader diversity of species and evolutionary context. Arabidopsis thaliana has been the primary model organism for plants, benefiting from a high-quality reference genome sequence and resources for reverse genetics. However, recent studies have made a group of species also in the Brassicaceae family and closely related to A. thaliana a focal point for comparative molecular, genomic, phenotypic and evolutionary studies. In this Review, we emphasize how such studies complement continued study of the model plant itself, provide an evolutionary perspective and summarize our current understanding of genetic and phenotypic diversity in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Karl, R. & Koch, M. A. A world-wide perspective on crucifer speciation and evolution: phylogenetics, biogeography and trait evolution in tribe Arabideae. Ann. Bot. 112, 983–1001 (2013).
O'Kane, S. L. & Al-Shehbaz, I. A. A synopsis of Arabidopsis (Brassicaceae). Novon 7, 323–327 (1997).
Koch, M., Haubold, B. & Mitchell-Olds, T. Molecular systematics of the Brassicaceae: evidence from coding plastidic matK and nuclear Chs sequences. Am. J. Bot. 88, 534–544 (2001).
Beilstein, M. A., Nagalingum, N. S., Clements, M. D., Manchester, S. R. & Mathews, S. Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 107, 18724–18728 (2010).
Bailey, C. D. et al. Toward a global phylogeny of the Brassicaceae. Mol. Biol. Evol. 23, 2142–2160 (2006).
Al-Shehbaz, I. A., Beilstein, M. A. & Kellogg, E. A. Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Plant Systemat Evol. 259, 89–120 (2006).
Couvreur, T. L. et al. Molecular phylogenetics, temporal diversification, and principles of evolution in the mustard family (Brassicaceae). Mol. Biol. Evol. 27, 55–71 (2010).
Franzke, A., Lysak, M. A., Al-Shehbaz, I. A., Koch, M. A. & Mummenhoff, K. Cabbage family affairs: the evolutionary history of Brassicaceae. Trends Plant Sci. 16, 108–116 (2011). This is a very readable, pleasantly non- Arabidopsis -centric summary of evolutionary trends in the Brassicaceae.
Kiefer, M. et al. BrassiBase: introduction to a novel knowledge database on brassicaceae evolution. Plant Cell Physiol. 55, e3 (2014).
Al-Shehbaz, I. A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 61, 931–954 (2012).
Lysak, M. A. Live and let die: centromere loss during evolution of plant chromosomes. New Phytol. 203, 1082–1089 (2014).
Lysak, M. A., Koch, M. A., Beaulieu, J. M., Meister, A. & Leitch, I. J. The dynamic ups and downs of genome size evolution in Brassicaceae. Mol. Biol. Evol. 26, 85–98 (2009).
Schranz, M. E., Lysak, M. A. & Mitchell-Olds, T. The ABC's of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci. 11, 535–542 (2006). This review provides a simple system of 24 conserved chromosomal blocks as a foundation for understanding genome evolution in the Brassicaceae.
Oyama, R. K. et al. The shrunken genome of Arabidopsis thaliana. Plant Systemat. Evol. 273, 257–271 (2008).
Ahmad, S. et al. Genetic dissection of basal defence responsiveness in accessions of Arabidopsis thaliana. Plant Cell Environ. 34, 1191–1206 (2011).
Koch, M. A. & Kiefer, M. Genome evolution among cruciferous plants: a lecture from the comparison of the genetic maps of three diploid species — Capsella rubella, Arabidopsis lyrata subsp. petraea, and A. thaliana. Am. J. Bot. 92, 761–767 (2005).
Slotte, T. et al. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nature Genet. 45, 831–835 (2013).
Haudry, A. et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nature Genet. 45, 891–898 (2013). This paper represents a major milestone in Brassicaceae comparative genomics, identifying tens of thousands of potential regulatory sequences from the combined analysis of nine genome assemblies.
Piegu, B. et al. Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 16, 1262–1269 (2006).
Long, Q. et al. Massive genomic variation and strong selection in Arabidopsis thaliana lines from Sweden. Nature Genet. 45, 884–890 (2013).
Seymour, D. K., Koenig, D., Hagmann, J., Becker, C. & Weigel, D. Evolution of DNA methylation patterns in the Brassicaceae is driven by differences in genome organization. PLoS Genet. 10, e1004785 (2014). This paper reports that there is little conservation of DNA methylation in the Brassicaceae family, even between closely related species, because most methylated sequences are evolutionarily short lived.
Willing, E.-M. et al. Genome expansion of Arabis alpina linked with retrotransposition and reduced symmetric DNA methylation. Nature Plants 1, 14023 (2015).
Dassanayake, M. et al. The genome of the extremophile crucifer Thellungiella parvula. Nature Genet. 43, 913–918 (2011).
Wu, H. J. et al. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc. Natl Acad. Sci. USA 109, 12219–12224 (2012).
Yang, R. L. et al. The reference genome of the halophytic plant Eutrema salsugineum. Front. Plant Sci. 4, 46 (2013).
Otto, S. P. The evolutionary consequences of polyploidy. Cell 131, 452–462 (2007).
Comai, L. The advantages and disadvantages of being polyploid. Nature Rev. Genet. 6, 836–846 (2005).
Marhold, K. & Lihova, J. Polyploidy, hybridization and reticulate evolution: lessons from the Brassicaceae. Plant Syst. Evol. 259, 143–174 (2006).
Joly, S., Heenan, P. B. & Lockhart, P. J. A. Pleistocene inter-tribal allopolyploidization event precedes the species radiation of Pachycladon (Brassicaceae) in New Zealand. Mol. Phylogenet. Evol. 51, 365–372 (2009). This study presents evidence for an apparent allopolyploidization event between two unknown species that belonged to two different lineages in the Brassicaceae.
Blanc, G., Barakat, A., Guyot, R., Cooke, R. & Delseny, M. Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12, 1093–1102 (2000).
Vision, T. J., Brown, D. G. & Tanksley, S. D. The origins of genomic duplications in Arabidopsis. Science 290, 2114–2117 (2000).
Simillion, C., Vandepoele, K., Van Montagu, M. C., Zabeau, M. & Van de Peer, Y. The hidden duplication past of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 99, 13627–13632 (2002).
Schranz, M. E., Kantama, L., de Jong, H. & Mitchell-Olds, T. Asexual reproduction in a close relative of Arabidopsis: a genetic investigation of apornixis in Boechera (Brassicaceae). New Phytol. 171, 425–438 (2006).
Barker, M. S., Vogel, H. & Schranz, M. E. Paleopolyploidy in the Brassicales: analyses of the Cleome transcriptome elucidate the history of genome duplications in Arabidopsis and other Brassicales. Genome Biol. Evol. 1, 391–399 (2009).
Mayrose, I. et al. Recently formed polyploid plants diversify at lower rates. Science 333, 1257 (2011).
Comai, L. et al. Phenotypic instability and rapid gene silencing in newly formed Arabidopsis allotetraploids. Plant Cell 12, 1551–1567 (2000).
Wang, J. et al. Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics 172, 507–517 (2006).
Schranz, M. E. & Osborn, T. C. Novel flowering time variation in the resynthesized polyploid Brassica napus. J. Hered. 91, 242–246 (2000).
Schranz, M. E. & Osborn, T. C. De novo variation in life-history traits and responses to growth conditions of resynthesized polyploid Brassica napus (Brassicaceae). Am. J. Bot. 91, 174–183 (2004).
Lee, H. S. & Chen, Z. J. Protein-coding genes are epigenetically regulated in Arabidopsis polyploids. Proc. Natl Acad. Sci. USA 98, 6753–6758 (2001).
Ha, M. et al. Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids. Proc. Natl Acad. Sci. USA 106, 17835–17840 (2009). This study implicates small RNAs as a major factor in the reconciliation of gene expression programmes after allopolyploidization.
Scannell, D. R., Byrne, K. P., Gordon, J. L., Wong, S. & Wolfe, K. H. Multiple rounds of speciation associated with reciprocal gene loss in polyploid yeasts. Nature 440, 341–345 (2006).
Bikard, D. et al. Divergent evolution of duplicate genes leads to genetic incompatibilities within A. thaliana. Science 323, 623–626 (2009).
Song, K., Lu, P., Tang, K. & Osborn, T. C. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proc. Natl Acad. Sci. USA 92, 7719–7723 (1995).
Xiong, Z., Gaeta, R. T. & Pires, J. C. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc. Natl Acad. Sci. USA 108, 7908–7913 (2011). This paper reports an amazingly fluid genome after allopolyploidization, with rampant aneuploidy, intergenomic and intragenomic rearrangements, chromosome breakage and fusion, rDNA changes and loss of repeat sequences.
Henry, I. M. et al. The BOY NAMED SUE quantitative trait locus confers increased meiotic stability to an adapted natural allopolyploid of Arabidopsis. Plant Cell 26, 181–194 (2014).
Yant, L. et al. Meiotic adaptation to genome duplication in Arabidopsis arenosa. Curr. Biol. 23, 2151–2156 (2013).
Hollister, J. D. et al. Genetic adaptation associated with genome-doubling in autotetraploid Arabidopsis arenosa. PLoS Genet. 8, e1003093 (2012).
Wendel, J. F. Genome evolution in polyploids. Plant Mol. Biol. 42, 225–249 (2000).
Wang, X. et al. The genome of the mesopolyploid crop species Brassica rapa. Nature Genet. 43, 1035–1039 (2011).
Liu, S. et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nature Commun. 5, 3930 (2014).
Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom (John Murray, 1876).
Charlesworth, D. & Charlesworth, B. The evolution and breakdown of S-allele systems. Heredity 43, 41–55 (1979).
Baker, H. Self-compatibility and establishment after 'long-distance' dispersal. Evolution 9, 347–349 (1955).
Stebbins, G. Self fertilization and population variability in the higher plants. Am. Nat. 91, 337–354 (1957).
Hedrick, P. W. Adaptive introgression in animals: examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 22, 4606–4618 (2013).
Bateman, A. J. Self-incompatibility systems in angiosperms: III. Cruciferae. Heredity 9, 53–68 (1955).
Takayama, S. & Isogai, A. Self-incompatibility in plants. Annu. Rev. Plant Biol. 56, 467–489 (2005).
Ivanov, R., Fobis-Loisy, I. & Gaude, T. When no means no: guide to Brassicaceae self-incompatibility. Trends Plant Sci. 15, 387–394 (2010).
Chantha, S. C., Herman, A. C., Platts, A. E., Vekemans, X. & Schoen, D. J. Secondary evolution of a self-incompatibility locus in the Brassicaceae genus Leavenworthia. PLoS Biol. 11, e1001560 (2013). This report describes a neofunctionalization event resulting in de novo evolution of self-incompatibility.
Tang, C. et al. The evolution of selfing in Arabidopsis thaliana. Science 317, 1070–1072 (2007).
Sherman-Broyles, S. et al. S locus genes and the evolution of self-fertility in Arabidopsis thaliana. Plant Cell 19, 94–106 (2007).
Guo, Y. L. et al. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proc. Natl Acad. Sci. USA 106, 5246–5251 (2009).
Foxe, J. P. et al. Recent speciation associated with the evolution of selfing in Capsella. Proc. Natl Acad. Sci. USA 106, 5241–5245 (2009). References 63 and 64 report on the recent speciation and evolution of self-compatibility of C. rubella.
Busch, J. W., Joly, S. & Schoen, D. J. Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Mol. Biol. Evol. 28, 1717–1729 (2011).
Tsuchimatsu, T., Kaiser, P., Yew, C. L., Bachelier, J. B. & Shimizu, K. K. Recent loss of self-incompatibility by degradation of the male component in allotetraploid Arabidopsis kamchatica. PLoS Genet. 8, e1002838 (2012).
Shimizu, K. K., Shimizu-Inatsugi, R., Tsuchimatsu, T. & Purugganan, M. D. Independent origins of self-compatibility in Arabidopsis thaliana. Mol. Ecol. 17, 704–714 (2008).
Nasrallah, J. B., Liu, P., Sherman-Broyles, S., Schmidt, R. & Nasrallah, M. E. Epigenetics mechanisms for breakdown of self-incompatibility in inter-specific hybrids. Genetics 175, 1965–1973 (2007).
Schmickl, R., Jorgensen, M. H., Brysting, A. K. & Koch, M. A. The evolutionary history of the Arabidopsis lyrata complex: a hybrid in the amphi-Beringian area closes a large distribution gap and builds up a genetic barrier. BMC Evol. Biol. 10, 98 (2010).
Koch, M. A. & Matschinger, M. Evolution and genetic differentiation among relatives of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 6272–6277 (2007).
Ross-Ibarra, J. et al. Patterns of polymorphism and demographic history in natural populations of Arabidopsis lyrata. PLoS ONE 3, e2411 (2008).
Wright, S. I., Lauga, B. & Charlesworth, D. Subdivision and haplotype structure in natural populations of Arabidopsis lyrata. Mol. Ecol. 12, 1247–1263 (2003).
Clauss, M. J. & Mitchell-Olds, T. Population genetic structure of Arabidopsis lyrata in Europe. Mol. Ecol. 15, 2753–2766 (2006).
Mable, B. K., Robertson, A. V., Dart, S., Di Berardo, C. & Witham, L. Breakdown of self-incompatibility in the perennial Arabidopsis lyrata (Brassicaceae) and its genetic consequences. Evolution 59, 1437–1448 (2005).
Griffin, P. C. & Willi, Y. Evolutionary shifts to self-fertilisation restricted to geographic range margins in North American Arabidopsis lyrata. Ecol. Lett. 17, 484–490 (2014).
Willi, Y. & Määttänen, K. The relative importance of factors determining genetic drift: mating system, spatial genetic structure, habitat and census size in Arabidopsis lyrata. New Phytol. 189, 1200–1209 (2011).
Willi, Y. & Maattanen, K. Evolutionary dynamics of mating system shifts in Arabidopsis lyrata. J. Evol. Biol. 23, 2123–2131 (2010).
Hoebe, P. N., Stift, M., Tedder, A. & Mable, B. K. Multiple losses of self-incompatibility in North-American Arabidopsis lyrata?: Phylogeographic context and population genetic consequences. Mol. Ecol. 18, 4924–4939 (2009).
Foxe, J. P. et al. Reconstructing origins of loss of self-incompatibility and selfing in North American Arabidopsis lyrata: a population genetic context. Evolution 64, 3495–3510 (2010).
Brandvain, Y., Slotte, T., Hazzouri, K. M., Wright, S. I. & Coop, G. Genomic identification of founding haplotypes reveals the history of the selfing species Capsella rubella. PLoS Genet. 9, e1003754 (2013).
Slotte, T., Hazzouri, K. M., Stern, D., Andolfatto, P. & Wright, S. I. Genetic architecture and adaptive significance of the selfing syndrome in Capsella. Evolution 66, 1360–1374 (2012).
St Onge, K. R., Kallman, T., Slotte, T., Lascoux, M. & Palme, A. E. Contrasting demographic history and population structure in Capsella rubella and Capsella grandiflora, two closely related species with different mating systems. Mol. Ecol. 20, 3306–3320 (2011).
Hurka, H., Friesen, N., German, D. A., Franzke, A. & Neuffer, B. 'Missing link' species Capsella orientalis and Capsella thracica elucidate evolution of model plant genus Capsella (Brassicaceae). Mol. Ecol. 21, 1223–1238 (2012).
Vekemans, X., Poux, C., Goubet, P. M. & Castric, V. The evolution of selfing from outcrossing ancestors in Brassicaceae: what have we learned from variation at the S-locus? J. Evolution Biol. 27, 1372–1385 (2014).
Charlesworth, D. & Willis, J. H. The genetics of inbreeding depression. Nature Rev. Genet. 10, 783–796 (2009).
Sicard, A. & Lenhard, M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann. Bot. Lond. 107, 1433–1443 (2011).
Sicard, A. et al. Genetics, evolution, and adaptive significance of the selfing syndrome in the genus Capsella. Plant Cell 23, 3156–3171 (2011). References 81 and 87 report that different strains of C. rubella share several loci for selfing-associated traits, suggesting that these evolved early during the species' history.
Böcher, T. W. Cytological and Embryological Studies in the Amphi-Apomictic Arabis holboellii Complex (Ejnar Minksgaard, 1951).
Koch, M., Al-Shehbaz, I. A. & Mummenhoff, K. Molecular systematics, evolution, and population biology in the mustard family (Brassicaceae). Ann. Mo. Bot. Gard. 90, 151–171 (2003).
Rushworth, C. A., Song, B. H., Lee, C. R. & Mitchell-Olds, T. Boechera, a model system for ecological genomics. Mol. Ecol. 20, 4843–4857 (2011).
Sharbel, T. F., Mitchell-Olds, T. M., Dobes, C., Kantama, L. & de Jong, H. Biogeographic distribution of polyploidy and B chromosomes in the apomictic Boechera holboellii complex. Cytogenet. Genome Res. 109, 283–292 (2005).
Kantama, L. et al. Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proc. Natl Acad. Sci. USA 104, 14026–14031 (2007).
Mau, M. et al. The conserved chimeric transcript UPGRADE2 is associated with unreduced pollen formation and is exclusively found in apomictic Boechera species. Plant Physiol. 163, 1640–1659 (2013).
Corral, J. M. et al. A conserved apomixis-specific polymorphism is correlated with exclusive exonuclease expression in premeiotic ovules of apomictic Boechera species. Plant Physiol. 163, 1660–1672 (2013). References 93 and 94 report the first molecular factors that were identified to be involved in natural apomixis.
Champagne, C. & Sinha, N. Compound leaves: equal to the sum of their parts? Development 131, 4401–4412 (2004).
Piazza, P. et al. Arabidopsis thaliana leaf form evolved via loss of KNOX expression in leaves in association with a selective sweep. Curr. Biol. 20, 2223–2228 (2010). This study suggests that the simple leaf form of A. thaliana arose by relatively recent strong selection.
Hay, A. S. et al. Cardamine hirsuta: a versatile genetic system for comparative studies. Plant J. 78, 1–15 (2014).
Hay, A. & Tsiantis, M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nature Genet. 38, 942–947 (2006).
Bharathan, G. et al. Homologies in leaf form inferred from KNOXI gene expression during development. Science 296, 1858–1860 (2002).
Long, J. A., Moan, E. I., Medford, J. I. & Barton, M. K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69 (1996).
Royer, D. L., Wilf, P., Janesko, D. A., Kowalski, E. A. & Dilcher, D. L. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. Am. J. Bot. 92, 1141–1151 (2005).
Barkoulas, M., Hay, A., Kougioumoutzi, E. & Tsiantis, M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genet. 40, 1136–1141 (2008).
Blein, T. et al. A conserved molecular framework for compound leaf development. Science 322, 1835–1839 (2008).
Rubio-Somoza, I. et al. Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr. Biol. 24, 2714–2719 (2014).
Nakayama, H. et al. Regulation of the KNOX-GA gene module induces heterophyllic alteration in North American lake cress. Plant Cell 26, 4733–4748 (2014). This paper reveals the molecular mechanisms underlying intra-individual differences in leaf shape in a non-model species.
Vlad, D. et al. Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science 343, 780–783 (2014). This study describes the genetic basis for leaf complexity differences among Arabidopsis relatives and highlights the importance of gene duplications during morphological evolution.
Saddic, L. A. et al. The LEAFY target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of CAULIFLOWER. Development 133, 1673–1682 (2006).
Sicard, A. et al. Repeated evolutionary changes of leaf morphology caused by mutations to a homeobox gene. Curr. Biol. 24, 1880–1886 (2014).
Kougioumoutzi, E. et al. SIMPLE LEAF3 encodes a ribosome-associated protein required for leaflet development in Cardamine hirsuta. Plant J. 73, 533–545 (2013).
Weigel, D. Natural variation in Arabidopsis: from molecular genetics to ecological genomics. Plant Physiol. 158, 2–22 (2012).
Guo, Y. L., Todesco, M., Hagmann, J., Das, S. & Weigel, D. Independent FLC mutations as causes of flowering-time variation in Arabidopsis thaliana and Capsella rubella. Genetics 192, 729–739 (2012).
Kuittinen, H., Niittyvuopio, A., Rinne, P. & Savolainen, O. Natural variation in Arabidopsis lyrata vernalization requirement conferred by a FRIGIDA indel polymorphism. Mol. Biol. Evol. 25, 319–329 (2008).
Wang, R. H. et al. PEP1 regulates perennial flowering in Arabis alpina. Nature 459, 423–U138 (2009). A gene controlling the singular onset of flowering in A. thaliana is required for cycles of flowering and vegetative growth in A. alpina.
Albani, M. C. et al. PEP1 of Arabis alpina is encoded by two overlapping genes that contribute to natural genetic variation in perennial flowering. PLoS Genet. 8, e1003130 (2012).
Bergonzi, S. et al. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 340, 1094–1097 (2013).
Wu, G. et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009).
Zhou, C. M. et al. Molecular basis of age-dependent vernalization in Cardamine flexuosa. Science 340, 1097–1100 (2013). References 115 and 117 describe different ways to 'rewire' regulatory networks controlling age- and temperature-dependent flowering.
Wang, J. W., Czech, B. & Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 (2009).
Melzer, S. et al. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genet. 40, 1489–1492 (2008).
Clauss, M. J. & Koch, M. A. Poorly known relatives of Arabidopsis thaliana. Trends Plant Sci. 11, 449–459 (2006).
Sasidharan, R. et al. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 163, 1277–1292 (2013).
Inan, G. et al. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 135, 1718–1737 (2004).
Turner, T. L., Bourne, E. C., Von Wettberg, E. J., Hu, T. T. & Nuzhdin, S. V. Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nature Genet. 42, 260–263 (2010). This paper reports one of the first uses of short-read resequencing to detect candidates for adaptation in a wild plant.
Kramer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 61, 517–534 (2010).
Verbruggen, N., Hermans, C. & Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 181, 759–776 (2009).
Kazemi-Dinan, A., Thomaschky, S., Stein, R. J., Krämer, U. & Müller, C. Zinc and cadmium hyperaccumulation act as deterrents towards specialist herbivores and impede the performance of a generalist herbivore. New Phytol. 202, 628–639 (2014).
Becher, M., Talke, I. N., Krall, L. & Kramer, U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 37, 251–268 (2004). The innovative use of microarrays in a close relative of A. thaliana revealed broad trends in gene expression shifts linked to metal hyperaccumulation.
Talke, I. N., Hanikenne, M. & KrÄmer, U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 142, 148–167 (2006).
Weber, M., Harada, E., Vess, C., Roepenack-Lahaye, E. & Clemens, S. Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 37, 269–281 (2004).
Willems, G. et al. Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytol. 187, 368–379 (2010).
Courbot, M. et al. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol. 144, 1052–1065 (2007).
Willems, G. et al. The genetic basis of zinc tolerance in the metallophyte Arabidopsis halleri ssp. halleri (Brassicaceae): an analysis of quantitative trait loci. Genetics 176, 659–674 (2007).
Frerot, H. et al. Genetic architecture of zinc hyperaccumulation in Arabidopsis halleri: the essential role of QTL x environment interactions. New Phytol. 187, 355–367 (2010).
Hanikenne, M. et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–395 (2008). This report describes how a series of changes were needed to increase expression of a transporter, HMA4, that is required for metal hyperaccumulation.
Hanikenne, M. et al. Hard selective sweep and ectopic gene conversion in a gene cluster affording environmental adaptation. PLoS Genet. 9, e1003707 (2013).
Craciun, A. R. et al. Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation. J. Exp. Bot. 63, 4179–4189 (2012).
Deinlein, U. et al. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 24, 708–723 (2012).
Shahzad, Z. et al. The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri. PLoS Genet. 6, e1000911 (2010).
Mirouze, M. et al. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 47, 329–342 (2006).
Roux, C. et al. Does speciation between Arabidopsis halleri and Arabidopsis lyrata coincide with major changes in a molecular target of adaptation? PLoS ONE 6, e26872 (2011).
Weinig, C., Ewers, B. E. & Welch, S. M. Ecological genomics and process modeling of local adaptation to climate. Curr. Opin. Plant Biol. 18, 66–72 (2014).
Kliebenstein, D. J., Kroymann, J. & Mitchell-Olds, T. The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 8, 264–271 (2005).
Schranz, M. E., Manzaneda, A. J., Windsor, A. J., Clauss, M. J. & Mitchell-Olds, T. Ecological genomics of Boechera stricta: identification of a QTL controlling the allocation of methionine- versus branched-chain amino acid-derived glucosinolates and levels of insect herbivory. Heredity 102, 465–474 (2009).
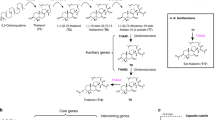
Prasad, K. V. et al. A gain-of-function polymorphism controlling complex traits and fitness in nature. Science 337, 1081–1084 (2012). This paper describes a prime example of biochemical innovation through gene duplication and divergent selection.
Reintanz, B. et al. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 13, 351–367 (2001).
Chen, S. X. et al. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J. 33, 923–937 (2003).
Koch, M. A. et al. BrassiBase: tools and biological resources to study characters and traits in the Brassicaceae-version 1.1. Taxon 61, 1001–1009 (2012).
Lyons, E. & Freeling, M. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53, 661–673 (2008).
Goodstein, D. M. et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186 (2012).
Acknowledgements
The authors thank D. Seymour and four anonymous reviewers for their comments on this manuscript. The authors' work on phenotypic diversity in the Brassicaceae has been supported by a Human Frontier Science Program long-term fellowship (D.K.), Deutsche Forschungsgemeinschaft (German Research Foundation) Priority Program 1529 – 'Adaptomics' (WE 2897; D.W.) and the Max Planck Society (D.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Copy number variation
-
Local expansion or contraction through tandem duplication of single-copy sequences or deletion of recently duplicated sequences.
- Homeologous
-
Pertaining to ancestrally related genes or chromosomes that arose through duplication during polyploidization.
- Subfunctionalization
-
The partitioning of ancestral function between recently duplicated gene copies.
- Neofunctionalization
-
The acquisition of a new function by a paralogue that has arisen through gene duplication.
- Inbreeding depression
-
The loss of vigour resulting from mating between related individuals and commensurate loss of heterozygosity.
- Sporophytic genetic incompatibility
-
Incompatibility determined by the parental genotype (that is, the sporophyte).
- Haplotype
-
Genetic variants that are inherited together, usually because of physical linkage on the same chromosome.
- Balancing selection
-
The selective forces that maintain genetic variation for longer than expected by random chance. Well-known examples include negative frequency-dependent selection and overdominance.
- Selfing syndrome
-
The suite of floral modifications associated with the transition from outcrossing to selfing. Most changes are thought to result from reduced investment in pollinator attraction and increased selfing efficiency.
- Apomixis
-
A form of plant asexual reproduction via the formation of seeds.
- Life history
-
The timing of key events such as germination and flowering.
- Meristem
-
Stem cell reservoir in plants.
- Metalliferous soils
-
Soils with high levels of potentially toxic trace elements.
Rights and permissions
About this article
Cite this article
Koenig, D., Weigel, D. Beyond the thale: comparative genomics and genetics of Arabidopsis relatives. Nat Rev Genet 16, 285–298 (2015). https://doi.org/10.1038/nrg3883
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3883
This article is cited by
-
Common evolutionary trajectory of short life-cycle in Brassicaceae ruderal weeds
Nature Communications (2023)
-
The Arabidopsis effector-triggered immunity landscape is conserved in oilseed crops
Scientific Reports (2022)
-
An updated explanation of ancestral karyotype changes and reconstruction of evolutionary trajectories to form Camelina sativa chromosomes
BMC Genomics (2020)
-
Adaptive responses of amino acid metabolism to the combination of desiccation and low nitrogen availability in Sporobolus stapfianus
Planta (2019)
-
Re-exploration of U’s Triangle Brassica Species Based on Chloroplast Genomes and 45S nrDNA Sequences
Scientific Reports (2018)