Key Points
-
The highly pleiotropic Notch signalling pathway functions to control cell-fate determination in nearly every tissue and organ by influencing differentiation, proliferation or apoptotic events.
-
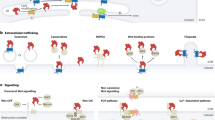
Notch pathway activity is highly dosage-sensitive and is regulated at different points along the signal transduction pathway by multiple mechanisms, including transcriptional control, endosomal trafficking and various post-translational modifications and microRNAs.
-
The developmental action of Notch links the fate of one cell to that of its immediate cellular neighbour, and recent genome-scale studies in Drosophila melanogaster indicate a complex network of hundreds of genes can affect Notch activity.
-
Multiple independent genome-wide screens in different developmental contexts in D. melanogaster indicate that a large proportion of the D. melanogaster genome is capable of affecting or modulating Notch activity and, by extension, other signalling pathways.
-
Positioning the vast number of genetic modifiers onto a single-protein complex map allows these genes to be connected into one unified network that provides a potential functional framework for modifying proteins in the context of all protein interactions within a cell, thereby generating numerous testable hypotheses.
Abstract
Notch signalling links the fate of one cell to that of an immediate neighbour and consequently controls differentiation, proliferation and apoptotic events in multiple metazoan tissues. Perturbations in this pathway activity have been linked to several human genetic disorders and cancers. Recent genome-scale studies in Drosophila melanogaster have revealed an extraordinarily complex network of genes that can affect Notch activity. This highly interconnected network contrasts our traditional view of the Notch pathway as a simple linear sequence of events. Although we now have an unprecedented insight into the way in which such a fundamental signalling mechanism is controlled by the genome, we are faced with serious challenges in analysing the underlying molecular mechanisms of Notch signal control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dexter, J. S. The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. Am. Nat. 48, 712–758 (1914).
Morgan, T. H. & Bridges, C. B. Sex-Linked Inheritance in Drosophila (Carnegie Institute of Washington, 1916).
Mohr, O. L. Character changes caused by mutation of an entire region of a chromosome in Drosophila. Genetics 4, 275–282 (1919).
Artavanis-Tsakonas, S. & Muskavitch, M. A. Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1–29 (2010).
Thorig, G. E., Heinstra, P. W. & Scharloo, W. The action of the notch locus in Drosophila melanogaster. II. Biochemical effects of recessive lethals on mitochondrial enzymes. Genetics 99, 65–74 (1981).
Thorig, G. E., Heinstra, P. W. & Scharloo, W. The action of the notch locus in Drosophila melanogaster. I. Effects of the Notch8 deficiency on mitochondrial enzymes. Mol. Gen. Genet. 182, 31–38 (1981).
Wharton, K. A., Johansen, K. M., Xu, T. & Artavanis-Tsakonas, S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43, 567–581 (1985). This was the original description of the molecular cloning and structure of the Notch receptor in D. melanogaster that revealed an epidermal growth factor (EGF)-repeat-containing transmembrane protein, suggesting a role for Notch in intracellular communication.
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nature Rev. Mol. Cell Biol. 7, 678–689 (2006).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Kopan, R. (ed.) Notch Signaling 1st edn (Academic Press, 2010).
Fortini, M. E. Introduction—Notch in development and disease. Semin. Cell Dev. Biol. 23, 419–420 (2012).
Bigas, A. & Espinosa, L. Hematopoietic stem cells: to be or Notch to be. Blood 119, 3226–3235 (2012).
Liu, J., Sato, C., Cerletti, M. & Wagers, A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 92, 367–409 (2010).
Artavanis-Tsakonas, S. The molecular biology of the Notch locus and the fine tuning of differentiation in Drosophila. Trends Genet. 4, 95–100 (1988).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Gridley, T. Notch signaling and inherited disease syndromes. Hum. Mol. Genet. 12 (Suppl. 1), R9–R13 (2003).
Louvi, A. & Artavanis-Tsakonas, S. Notch and disease: a growing field. Semin. Cell Dev. Biol. 23, 473–480 (2012).
Ranganathan, P., Weaver, K. L. & Capobianco, A. J. Notch signalling in solid tumours: a little bit of everything but not all the time. Nature Rev. Cancer 11, 338–351 (2011).
Dorer, D. R. & Christensen, A. C. A recombinational hotspot at the triplo-lethal locus of Drosophila melanogaster. Genetics 122, 397–401 (1989).
Mazzone, M. et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc. Natl Acad. Sci. USA 107, 5012–5017 (2010).
Oda, T. et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nature Genet. 16, 235–242 (1997).
McDaniell, R. et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 79, 169–173 (2006).
Garg, V. et al. Mutations in NOTCH1 cause aortic valve disease. Nature 437, 270–274 (2005).
Park, J. T. et al. Notch3 gene amplification in ovarian cancer. Cancer Res. 66, 6312–6318 (2006).
Lee, S. Y. et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci. 100, 920–926 (2009).
Roy, M., Pear, W. S. & Aster, J. C. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev. 17, 52–59 (2007).
Greenwald, I. & Rubin, G. M. Making a difference: the role of cell–cell interactions in establishing separate identities for equivalent cells. Cell 68, 271–281 (1992).
Seugnet, L., Simpson, P. & Haenlin, M. Transcriptional regulation of Notch and Delta: requirement for neuroblast segregation in Drosophila. Development 124, 2015–2025 (1997). This study evaluated the role of transcriptional regulation during lateral inhibition within the proneural group and how one cell overcomes Notch-mediated repression.
Kooh, P. J., Fehon, R. G. & Muskavitch, M. A. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development 117, 493–507 (1993).
Kopczynski, C. C. & Muskavitch, M. A. Complex spatio-temporal accumulation of alternative transcripts from the neurogenic gene Delta during Drosophila embryogenesis. Development 107, 623–636 (1989).
D'Souza, B., Meloty-Kapella, L. & Weinmaster, G. Canonical and non-canonical Notch ligands. Curr. Top. Dev. Biol. 92, 73–129 (2010).
Fehon, R. G. et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61, 523–534 (1990). This paper was the first to show that the Notch and Delta proteins physically interact at the cell surface through their extracellular domains in a calcium-dependent manner.
Sprinzak, D. et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86–90 (2010). This study showed that cis interaction of Notch–Delta generates an ultrasensitive switch between mutually exclusive (sending versus receiving) signalling states and results in the amplification of small differences in expression levels between neighbouring cells.
Glittenberg, M., Pitsouli, C., Garvey, C., Delidakis, C. & Bray, S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. EMBO J. 25, 4697–4706 (2006).
Cordle, J. et al. A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nature Struct. Mol. Biol. 15, 849–857 (2008).
Kankel, M. W. et al. Investigating the genetic circuitry of mastermind in Drosophila, a notch signal effector. Genetics 177, 2493–2505 (2007). This was the first large-scale genetic screen for Notch pathway modifiers in D. melanogaster using the Exelixis mutant collection, more than doubling the number of genes known to interact with Notch, revealing that a highly complex network of genes and functionalities are involved in mediating Notch activity.
Shalaby, N. A. et al. A screen for modifiers of notch signaling uncovers Amun, a protein with a critical role in sensory organ development. Genetics 182, 1061–1076 (2009).
Saj, A. et al. A combined ex vivo and in vivo RNAi screen for notch regulators in Drosophila reveals an extensive Notch interaction network. Dev. Cell 18, 862–876 (2010). A comprehensive, genome-wide cell-based RNAi screen dissecting Notch regulation and its connections to cellular pathways identified candidate Notch regulators. Many candidates were validated in vivo by using transgenic D. melanogaster RNAi strains.
Mummery-Widmer, J. L. et al. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458, 987–992 (2009). This was the first genome-wide RNAi screen for Notch pathway modifiers during external sensory organ development in D. melanogaster , uncovering hundreds of genes involved in lateral inhibition and asymmetric cell division.
Kovall, R. A. & Blacklow, S. C. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr. Top. Dev. Biol. 92, 31–71 (2010).
Stanley, P. & Okajima, T. Roles of glycosylation in Notch signaling. Curr. Top. Dev. Biol. 92, 131–164 (2010).
Le Bras, S., Loyer, N. & Le Borgne, R. The multiple facets of ubiquitination in the regulation of notch signaling pathway. Traffic 12, 149–161 (2011).
Fortini, M. E. Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16, 633–647 (2009).
Staub, O. & Rotin, D. Role of ubiquitylation in cellular membrane transport. Physiol. Rev. 86, 669–707 (2006).
Yamamoto, S., Charng, W. L. & Bellen, H. J. Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top. Dev. Biol. 92, 165–200 (2010).
Bernard, F., Krejci, A., Housden, B., Adryan, B. & Bray, S. J. Specificity of Notch pathway activation: twist controls the transcriptional output in adult muscle progenitors. Development 137, 2633–2642 (2010).
Wang, H. et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc. Natl Acad. Sci. USA 108, 14908–14913 (2011).
Li, Y., Hibbs, M. A., Gard, A. L., Shylo, N. A. & Yun, K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 30, 741–752 (2012).
Artavanis-Tsakonas, S. Accessing the Exelixis collection. Nature Genet. 36, 207 (2004).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Mourikis, P., Lake, R. J., Firnhaber, C. B. & DeDecker, B. S. Modifiers of notch transcriptional activity identified by genome-wide RNAi. BMC Dev. Biol. 10, 107 (2010).
Lai, E. C., Tam, B. & Rubin, G. M. Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs. Genes Dev. 19, 1067–1080 (2005).
Wang, Z. et al. Cross-talk between miRNA and Notch signaling pathways in tumor development and progression. Cancer Lett. 292, 141–148 (2010).
Inui, M., Martello, G. & Piccolo, S. MicroRNA control of signal transduction. Nature Rev. Mol. Cell Biol. 11, 252–263 (2010).
Ichimura, A., Ruike, Y., Terasawa, K. & Tsujimoto, G. miRNAs and regulation of cell signaling. FEBS J. 278, 1610–1618 (2011).
Greenwald, I. LIN-12/Notch signaling in C. elegans. WormBook 8 Aug 2005 (doi:10.1895/wormbook.1.10.1).
Levitan, D. & Greenwald, I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature 377, 351–354 (1995). By screening for suppressors of a Notch gain-of-function mutation in C. elegans , presenilin was identified as a regulator of Notch activity and was thus the first study to link the presenilin complex — which is implicated in Alzheimer's disease — with Notch signalling.
Kopan, R. & Goate, A. A common enzyme connects Notch signaling and Alzheimer's disease. Genes Dev. 14, 2799–2806 (2000).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Li, X. & Greenwald, I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl Acad. Sci. USA 94, 12204–12209 (1997).
Westlund, B., Parry, D., Clover, R., Basson, M. & Johnson, C. D. Reverse genetic analysis of Caenorhabditis elegans presenilins reveals redundant but unequal roles for sel-12 and hop-1 in Notch-pathway signaling. Proc. Natl Acad. Sci. USA 96, 2497–2502 (1999).
Kopan, R. & Ilagan, M. X. γ-secretase: proteasome of the membrane? Nature Rev. Mol. Cell Biol. 5, 499–504 (2004).
Lehner, B., Crombie, C., Tischler, J., Fortunato, A. & Fraser, A. G. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nature Genet. 38, 896–903 (2006).
Mukherjee, A. et al. Regulation of Notch signalling by non-visual β-arrestin. Nature Cell Biol. 7, 1191–1201 (2005).
Krauss, G. Biochemistry of Signal Transduction and Regulation 3rd edn (Wiley-VCH, 2003).
Hurlbut, G. D., Kankel, M. W. & Artavanis-Tsakonas, S. Nodal points and complexity of Notch–Ras signal integration. Proc. Natl Acad. Sci. USA 106, 2218–2223 (2009). A microarray-based approach indicating the integration of Notch signals with other major signalling pathways is described in this paper. Importantly, this study also showed that most genes that are responsive to RTK signalling are also responsive to Notch signalling, showing that the Notch and RTK pathways are highly interconnected.
Flaherty, M. S., Zavadil, J., Ekas, L. A. & Bach, E. A. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of Notch signaling by the JAK/STAT pathway. Dev. Dynam. 238, 2235–2253 (2009).
Krejci, A., Bernard, F., Housden, B. E., Collins, S. & Bray, S. J. Direct response to Notch activation: signalling crosstalk and incoherent logic. Sci. Signal. 2, ra1 (2009). This study catalogued the immediate cellular consequences of Notch activation in cultured cells using mRNA expression and CBF1–SU(H)–LAG1 (CSL) occupancy at enhancers.
Hegde, A. et al. Genomewide expression analysis in zebrafish Mind bomb alleles with pancreas defects of different severity identifies putative Notch responsive genes. PLoS ONE 3, e1479 (2008).
Hamidi, H., Gustafason, D., Pellegrini, M. & Gasson, J. Identification of novel targets of CSL-dependent Notch signaling in hematopoiesis. PLoS ONE 6, e20022 (2011).
South, A. P., Cho, R. J. & Aster, J. C. The double-edged sword of Notch signaling in cancer. Semin. Cell Dev. Biol. 23, 458–464 (2012).
Hurlbut, G. D., Kankel, M. W., Lake, R. J. & Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19, 166–175 (2007).
Fortini, M. E., Rebay, I., Caron, L. A. & Artavanis-Tsakonas, S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature 365, 555–557 (1993).
Rones, M. S., McLaughlin, K. A., Raffin, M. & Mercola, M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127, 3865–3876 (2000).
Yeo, S. Y. Zebrafish CiA interneurons are late-born primary neurons. Neurosci. Lett. 466, 131–134 (2009).
Sundaram, M. V. The love–hate relationship between Ras and Notch. Genes Dev. 19, 1825–1839 (2005).
Rebay, I. Keeping the receptor tyrosine kinase signaling pathway in check: lessons from Drosophila. Dev. Biol. 251, 1–17 (2002).
Doroquez, D. B. & Rebay, I. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit. Rev. Biochem. Mol. Biol. 41, 339–385 (2006).
Voas, M. G. & Rebay, I. Signal integration during development: insights from the Drosophila eye. Dev. Dynam. 229, 162–175 (2004).
Culi, J., Martin-Blanco, E. & Modolell, J. The EGF receptor and N signalling pathways act antagonistically in Drosophila mesothorax bristle patterning. Development 128, 299–308 (2001).
zur Lage, P. & Jarman, A. P. Antagonism of EGFR and notch signalling in the reiterative recruitment of Drosophila adult chordotonal sense organ precursors. Development 126, 3149–3157 (1999).
Carmena, A. et al. Reciprocal regulatory interactions between the Notch and Ras signaling pathways in the Drosophila embryonic mesoderm. Dev. Biol. 244, 226–242 (2002).
Price, J. V., Savenye, E. D., Lum, D. & Breitkreutz, A. Dominant enhancers of EGFR in Drosophila melanogaster: genetic links between the Notch and EGFR signaling pathways. Genetics 147, 1139–1153 (1997).
Sundaram, M. V. Vulval development: the battle between Ras and Notch. Curr. Biol. 14, R311–R313 (2004).
Charlton-Perkins, M. et al. Prospero and Pax2 combinatorially control neural cell fate decisions by modulating Ras- and Notch-dependent signaling. Neural Dev. 6, 20 (2011).
Roemer, K. Notch and the p53 clan of transcription factors. Adv. Exp. Med. Biol. 727, 223–240 (2012).
Dang, T. P. Notch, apoptosis and cancer. Adv. Exp. Med. Biol. 727, 199–209 (2012).
Guo, S., Liu, M. & Gonzalez-Perez, R. R. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim. Biophys. Acta 1815, 197–213 (2011).
Fuxe, J., Vincent, T. & Garcia de Herreros, A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle 9, 2363–2374 (2010).
Ristorcelli, E. & Lombardo, D. Targeting Notch signaling in pancreatic cancer. Expert Opin. Ther. Targets 14, 541–552 (2010).
Li, J. L. & Harris, A. L. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Frontiers Biosci. 14, 3094–3110 (2009).
Holderfield, M. T. & Hughes, C. C. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circul. Res. 102, 637–652 (2008).
Villaronga, M. A., Bevan, C. L. & Belandia, B. Notch signaling: a potential therapeutic target in prostate cancer. Curr. Cancer Drug Targets 8, 566–580 (2008).
Nakamura, T., Tsuchiya, K. & Watanabe, M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J. Gastroenterol. 42, 705–710 (2007).
Uetz, P. et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627 (2000).
Ito, T. et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl Acad. Sci. USA 98, 4569–4574 (2001).
Giot, L. et al. A protein interaction map of Drosophila melanogaster. Science 302, 1727–1736 (2003). This study presented the first and the largest binary interaction (yeast-two hybrid) map for D. melanogaster . This work marks one of the earliest efforts at modelling multicellular organisms using a systems-biology approach.
Li, S. et al. A map of the interactome network of the metazoan C. elegans. Science 303, 540–543 (2004).
Stelzl, U. et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell 122, 957–968 (2005).
Rual, J. F. et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature 437, 1173–1178 (2005).
Stanyon, C. A. et al. A Drosophila protein-interaction map centered on cell-cycle regulators. Genome Biol. 5, R96 (2004).
Krogan, N. J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006).
Ho, Y. et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 (2002).
Gavin, A. C. et al. Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 (2006).
Cramer, R. Editorial for “advances in biological mass spectrometry and proteomics”. Methods 54, 349–350 (2011).
Gavin, A. C., Maeda, K. & Kuhner, S. Recent advances in charting protein-protein interaction: mass spectrometry-based approaches. Curr. Opin. Biotechnol. 22, 42–49 (2011).
Bandyopadhyay, S., Kelley, R., Krogan, N. J. & Ideker, T. Functional maps of protein complexes from quantitative genetic interaction data. PLoS Comput. Biol. 4, e1000065 (2008).
Costanzo, M. et al. The genetic landscape of a cell. Science 327, 425–431 (2010).
van Wageningen, S. et al. Functional overlap and regulatory links shape genetic interactions between signaling pathways. Cell 143, 991–1004 (2010).
Li, B., Cao, W., Zhou, J. & Luo, F. Understanding and predicting synthetic lethal genetic interactions in Saccharomyces cerevisiae using domain genetic interactions. BMC Systems Biol. 5, 73 (2011).
Linden, R. O., Eronen, V. P. & Aittokallio, T. Quantitative maps of genetic interactions in yeast — comparative evaluation and integrative analysis. BMC Systems Biol. 5, 45 (2011).
Horn, T. et al. Mapping of signaling networks through synthetic genetic interaction analysis by RNAi. Nature Methods 8, 341–346 (2011).
Guruharsha, K. G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011). This study presents the first metazoan protein complex map and defined more than 500 protein complexes involving several thousand proteins.
Fan, X. et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 64, 7787–7793 (2004).
Heitzler, P. Biodiversity and noncanonical Notch signaling. Curr. Top. Dev. Biol. 92, 457–481 (2010).
Hu, Q. D. et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115, 163–175 (2003).
Cui, X. Y. et al. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J. Biol. Chem. 279, 25858–25865 (2004).
Hori, K. et al. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development 131, 5527–5537 (2004).
Shawber, C. et al. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 122, 3765–3773 (1996).
Rusconi, J. C. & Corbin, V. Evidence for a novel Notch pathway required for muscle precursor selection in Drosophila. Mech. Dev. 79, 39–50 (1998).
Martinez Arias, A., Zecchini, V. & Brennan, K. CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr. Opin. Genet. Dev. 12, 524–533 (2002).
Vaccari, T. & Bilder, D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687–698 (2005).
Thompson, B. J. et al. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 9, 711–720 (2005).
Vaccari, T., Lu, H., Kanwar, R., Fortini, M. E. & Bilder, D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755–762 (2008).
Vaccari, T. et al. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 122, 2413–2423 (2009).
Wilkin, M. B. et al. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14, 2237–2244 (2004).
Hori, K., Sen, A., Kirchhausen, T. & Artavanis-Tsakonas, S. Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J. Cell Biol. 195, 1005–1015 (2011).
Couturier, L., Vodovar, N. & Schweisguth, F. Endocytosis by Numb breaks Notch symmetry at cytokinesis. Nature Cell Biol. 14, 131–139 (2012).
Croft, D. et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 39, D691–D697 (2011).
Gordon, W. R. et al. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS ONE 4, e6613 (2009).
Lake, R. J., Grimm, L. M., Veraksa, A., Banos, A. & Artavanis-Tsakonas, S. In vivo analysis of the Notch receptor S1 cleavage. PLoS ONE 4, e6728 (2009).
Qurashi, A. et al. HSPC300 and its role in neuronal connectivity. Neural Dev. 2, 18 (2007).
Kanehisa, M., Goto, S., Furumichi, M., Tanabe, M. & Hirakawa, M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 38, D355–D360 (2010).
Warde-Farley, D. et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38, W214–W220 (2010).
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P. L. & Ideker, T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432 (2011).
Acknowledgements
We would like to thank A. Louvi, D. Dimlich, K. Hori, B. Obar and A. Sen for critically reading the manuscript. Special thanks to J. Iwasa for the Notch pathway animations. We also apologize to our colleagues whose original work was not discussed or cited here in the interest of space. Work in the Artavanis-Tsakonas laboratory is supported by grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
(MOV 5028 kb)
Supplementary information S2 (movie)
(MOV 1289 kb)
Supplementary information S3 (movie)
(MOV 3803 kb)
Supplementary information S4 (table)
(XLS 308 kb)
Supplementary information S5 (figure)
High-resolution image file from Figure 4: Mapping genetic modifiers on proteome map (PDF 7288 kb)
Related links
Glossary
- Haploinsufficiency
-
A genetic condition in a diploid organism in which a single functional copy of a gene fails to generate sufficient gene product, leading to an abnormal or diseased state.
- Triplomutant
-
A genetic variant that carries three copies of a single gene, as opposed to the normal two copies, and displays a specific mutant phenotype that may include lethality or morphological defects. This mutant is distinct from aneuploidy, which alters copy numbers for large numbers of genes owing to chromosomal aberrations.
- Alagille's syndrome
-
An inherited autosomal dominant genetic disorder that can affect multiple vital organs, such as the liver, heart and other body parts. The disorder may also affect the blood vessels within the brain, spinal cord and the kidneys. The estimated prevalence of Alagille's syndrome is 1 in 70,000 newborns.
- Transcriptional feedback
-
A regulatory loop in which the gene products positively or negatively regulate the expression or activity of other members of the same pathway and therefore regulate themselves.
- Equivalence group
-
A group of unspecified cells that have the same developmental potential to adopt various fates. Typically, these are cells from an equivalence group that receive a signal take on fates that are distinct from those cells that do not receive a signal and therefore adopt a default fate.
- Quantitative proteomics
-
Quantitative proteomics is identical to general (qualitative) proteomics but includes quantification as an additional dimension. Information about differences between two or more protein samples is obtained with the use of isotopes or mass tags that are distinguishable in mass spectrometry.
Rights and permissions
About this article
Cite this article
Guruharsha, K., Kankel, M. & Artavanis-Tsakonas, S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 13, 654–666 (2012). https://doi.org/10.1038/nrg3272
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3272
This article is cited by
-
Role of cell rearrangement and related signaling pathways in the dynamic process of tip cell selection
Cell Communication and Signaling (2024)
-
TM2D3, a mammalian homologue of Drosophila neurogenic gene product Almondex, regulates surface presentation of Notch receptors
Scientific Reports (2023)
-
Jag1-Notch cis-interaction determines cell fate segregation in pancreatic development
Nature Communications (2023)
-
The Circadian System Is Essential for the Crosstalk of VEGF-Notch-mediated Endothelial Angiogenesis in Ischemic Stroke
Neuroscience Bulletin (2023)
-
Impact of structural features of acetylated bacterial cellulose on cell-scaffold and scaffold-blood interactions in vitro
Cellulose (2023)