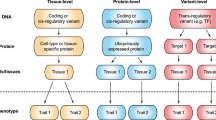
Abstract
Recent developments in the collection and analysis of cellular multilayered data in large cohorts with extensive organismal phenotyping promise to reveal links between genetic variation and biological processes. The use of these cellular resources as models for human biology — known as 'cellular phenotyping' — is likely to transform our understanding of the genetic and long-term environmental influences on complex traits. I discuss the advantages and caveats of a deeper analysis of cellular phenotypes in large cohorts and assess the methodological advances, resource needs and prospects of this new approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gibson, G. The environmental contribution to gene expression profiles. Nature Rev. Genet. 9, 575–581 (2008).
Idaghdour, Y., Storey, J. D., Jadallah, S. J. & Gibson, G. A genome-wide gene expression signature of environmental geography in leukocytes of Moroccan Amazighs. PLoS Genet. 4, e1000052 (2008).
Dermitzakis, E. T. From gene expression to disease risk. Nature Genet. 40, 492–493 (2008).
Cheung, V. G. et al. Mapping determinants of human gene expression by regional and genome-wide association. Nature 437, 1365–1369 (2005).
Stranger, B. E. et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 1, e78 (2005).
Foss, E. J. et al. Genetic basis of proteome variation in yeast. Nature Genet. 39, 1369–1375 (2007).
Choy, E. et al. Genetic analysis of human traits in vitro: drug response and gene expression in lymphoblastoid cell lines. PLoS Genet. 4, e1000287 (2008).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nature Genet. 40, 768–775 (2008).
Montgomery, S. B., Lappalainen, T., Gutierrez-Arcelus, M. & Dermitzakis, E. T. Rare and common regulatory variation in population-scale sequenced human genomes. PLoS Genet. 7, e1002144 (2011).
Caliskan, M., Cusanovich, D. A., Ober, C. & Gilad, Y. The effects of EBV transformation on gene expression levels and methylation profiles. Hum. Mol. Genet. 20, 1643–1652 (2011).
McDaniell, R. et al. Heritable individual-specific and allele-specific chromatin signatures in humans. Science 328, 235–239 (2010).
Yamanaka, S. Elite and stochastic models for induced pluripotent stem cell generation. Nature 460, 49–52 (2009).
Gore, A. et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471, 63–67 (2011).
Montgomery, S. B. & Dermitzakis, E. T. From expression QTLs to personalized transcriptomics. Nature Rev. Genet. 12, 277–282 (2011).
Kasowski, M. et al. Variation in transcription factor binding among humans. Science 328, 232–235 (2010).
Montgomery, S. B. et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464, 773–777 (2010).
Pickrell, J. K. et al. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature 464, 768–772 (2010).
Stranger, B. E. et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315, 848–853 (2007).
Gaulton, K. J. et al. A map of open chromatin in human pancreatic islets. Nature Genet. 42, 255–259 (2010).
Putoux, A. et al. KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nature Genet. 43, 601–606 (2011).
Kim, S. et al. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nature Cell Biol. 13, 351–360 (2011).
Duan, S. et al. Expression and alternative splicing of folate pathway genes in HapMap lymphoblastoid cell lines. Pharmacogenomics 10, 549–563 (2009).
Gamazon, E. R., Huang, R. S., Cox, N. J. & Dolan, M. E. Chemotherapeutic drug susceptibility associated SNPs are enriched in expression quantitative trait loci. Proc. Natl Acad. Sci. USA 107, 9287–9292 (2010).
Smirnov, D. A., Morley, M., Shin, E., Spielman, R. S. & Cheung, V. G. Genetic analysis of radiation-induced changes in human gene expression. Nature 459, 587–591 (2009).
Dimas, A. S. & Dermitzakis, E. T. Genetic variation of regulatory systems. Curr. Opin. Genet. Dev. 19, 586–590 (2009).
Emilsson, V. et al. Genetics of gene expression and its effect on disease. Nature 452, 423–428 (2008).
Schadt, E. E. Molecular networks as sensors and drivers of common human diseases. Nature 461, 218–223 (2009).
Schadt, E. E. et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nature Genet. 37, 710–717 (2005).
Mackay, T. F., Stone, E. A. & Ayroles, J. F. The genetics of quantitative traits: challenges and prospects. Nature Rev. Genet. 10, 565–577 (2009).
Suhre, K. et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011).
Bell, J. T. et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 12, R10 (2011).
Nica, A. C. et al. Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet. 6, e1000895 (2010).
Nicolae, D. L. et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 6, e1000888 (2010).
Lappalainen, T., Montgomery, S. B., Nica, A. C. & Dermitzakis, E. T. Epistatic selection between coding and regulatory variation in human evolution and disease. Am. J. Hum. Genet. 89, 459–463 (2011).
Bouzakri, K. et al. Bimodal effect on pancreatic β-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes 60, 1111–1121 (2011).
Acknowledgements
The author would like to thank the Louis-Jeantet Foundation and the Swiss National Science Foundation for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Additive
-
In the context of a genetic effect, the linear relationship between the replacement of an allele and its effect on the phenotype.
- Expression quantitative trait loci
-
(eQTLs). Loci at which genetic allelic variation is associated with variation in gene expression.
- Induced pluripotent stem cells
-
(iPSCs). Cells that are derived from somatic cells by 'reprogramming' or de-differentiation that is triggered by the transfection of pluripotency genes, which alters the somatic cells to a state that is similar to that of embryonic stem cells.
- Marginal effects
-
Also known as main effects, these are the effects of a variable assuming no dependency or conditionality of other variables.
- Metabolomics
-
The directed use of quantitative analytical methods for analysing the entire metabolic content of a cell or organism (that is, the metabolome) at a given time.
- Purifying natural selection
-
Natural selection that results in the reduction or elimination of the frequency of alleles with negative fitness effects.
Rights and permissions
About this article
Cite this article
Dermitzakis, E. Cellular genomics for complex traits. Nat Rev Genet 13, 215–220 (2012). https://doi.org/10.1038/nrg3115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3115
This article is cited by
-
Exploring the genetic basis of human population differences in DNA methylation and their causal impact on immune gene regulation
Genome Biology (2018)
-
Functional fingerprinting of human mesenchymal stem cells using high-throughput RNAi screening
Genome Medicine (2015)
-
Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics
Molecular Psychiatry (2015)
-
Genetics and Society—Educating Scientifically Literate Citizens: Introduction to the Thematic Issue
Science & Education (2014)