Key Points
-
The fitness effects of antibiotic-resistance mutations and of mutations that compensate for the cost of resistance depends on the molecular basis of resistance and the ecological (or treatment) context in which resistance evolves.
-
The distribution of fitness effects of resistance mutations is determined by antibiotic dose and drug–target interactions.
-
Resistance mutations impose a fitness cost that varies widely among mutations. It may be possible to predict costs of resistance by considering the effects of resistance mutations on protein stability.
-
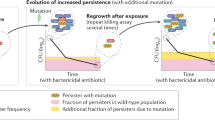
Compensatory mutations alleviate the cost of resistance, allowing resistant strains to persist in the absence of antibiotics. The opportunity for compensation varies among resistant mutants and it may be possible to predict this variability by explicitly considering the mechanistic basis of the costs of resistance.
-
Physiological interactions between antibiotics and genetic interactions between resistance mutations are crucial for the evolution of multidrug resistance by modifying the benefits associated with resistance and compensatory mutation.
-
Immigration from antibiotic-free populations is important in the evolution of resistance. Immigration accelerates the evolution of resistance when resistance mutations are rare and immigration can reverse resistance following the cessation of antibiotic use.
-
Spatial and temporal patterns of antibiotic use play a key part in the evolution of resistance. Resistance evolves most slowly under maximal levels of environmental heterogeneity.
-
Future work should concentrate on developing predictive models of resistance evolution by integrating molecular mechanisms of resistance with treatment context. This may help develop improved treatment strategies for preventing resistance evolution in pathogen populations.
Abstract
Despite efforts from a range of disciplines, our ability to predict and combat the evolution of antibiotic resistance in pathogenic bacteria is limited. This is because resistance evolution involves a complex interplay between the specific drug, bacterial genetics and both natural and treatment ecology. Incorporating details of the molecular mechanisms of drug resistance and ecology into evolutionary models has proved useful in predicting the dynamics of resistance evolution. However, putting these models to practical use will require extensive collaboration between mathematicians, molecular biologists, evolutionary ecologists and clinicians.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Palumbi, S. R. Evolution — humans as the world's greatest evolutionary force. Science 293, 1786–1790 (2001).
Maynard Smith, J., Feil, E. J. & Smith, N. H. Population structure and evolutionary dynamics of pathogenic bacteria. Bioessays 22, 1115–1122 (2000).
Levy, S. B. & Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Med. 10, S122–S129 (2004).
Lipsitch, M. The rise and fall of antimicrobial resistance. Trends Microbiol. 9, 438–444 (2001).
Levin, B. R., Perrot, V. & Walker, N. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154, 985–997 (2000).
Buckling, A., MacLean, R. C., Brockhurst, M. & Colegrave, N. The Beagle in a bottle. Nature 457, 824–829 (2009).
Gagneux, S. et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312, 1944–1946 (2006). This paper measures the cost of rifampicin resistance in M. tuberculosis and shows that the least costly resistance mutations are the most prevalent mutations in clinical populations of this important pathogen.
Nagaev, I., Bjorkman, J., Andersson, D. I. & Hughes, D. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 40, 433–439 (2001).
Rozen, D. E., McGee, L., Levin, B. R. & Klugman, K. P. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 51, 412–416 (2007).
MacLean, R. C. & Buckling, A. The distribution of fitness effects of beneficial mutations in Pseudomonas aeruginosa. PLoS Genet. 5, e1000406 (2009). This paper shows that the DFBM of rifampicin resistance mutations is predictably variable as a function of antibiotic dose, and highlights the importance of drug–target interactions for determining the DFBM.
Thomas, V. L., McReynolds, A. C. & Shoichet, B. K. Structural bases for stability–function tradeoffs in antibiotic resistance. J. Mol. Biol. 396, 47–59 (2010).
Wang, X. J., Minasov, G. & Shoichet, B. K. Evolution of an antibiotic resistance enzyme constrained by stability and activity trade-offs. J. Mol. Biol. 320, 85–95 (2002).
Weinreich, D. M., Delaney, N. F., DePristo, M. A. & Hartl, D. L. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312, 111–114 (2006).
Michel, J. B., Yeh, P. J., Chait, R., Moellering, R. C. & Kishony, R. Drug interactions modulate the potential for evolution of resistance. Proc.Natl Acad. Sci. USA 105, 14918–14923 (2008).
Yeh, P. J., Hegreness, M. J., Aiden, A. P. & Kishony, R. Drug interactions and the evolution of antibiotic resistance. Nature Rev. Microbiol. 7, 460–466 (2009). This excellent Review shows how physiological interactions among antibiotics play a key part in determining the rate of evolution of multidrug resistance.
Perron, G. G., Gonzalez, A. & Buckling, A. Source–sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness cost. Proc. R. Soc. Lond., B 274, 2351–2356 (2007).
Perron, G. G., Gonzalez, A. & Buckling, A. The rate of environmental change drives adaptation to an antibiotic sink. J. Evol. Biol. 21, 1724–1731 (2008).
Palmer, A. C., Angelino, E. & Kishony, R. Chemical decay of an antibiotic inverts selection for resistance. Nature Chem. Biol. 6, 105–107.
Bollenbach, T., Quan, S., Chait, R. & Kishony, R. Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell 139, 707–718 (2009).
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000).
Andersson, D. I. The biological cost of mutational antibiotic resistance: any practical conclusions? Curr. Opin. Microbiol. 9, 461–465 (2006).
Andersson, D. I. & Levin, B. R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2, 489–493 (1999).
Bjorkman, J., Hughes, D. & Andersson, D. I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc. Natl Acad. Sci. USA 95, 3949–3953 (1998).
Maisnier-Patin, S. & Andersson, D. I. Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res. Microbiol. 155, 360–369 (2004).
Andersson, D. I. & Hughes, D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nature Rev. Microbiol. 8, 260–271 (2010). This excellent Review provides a comprehensive summary of research on the cost of resistance, compensatory adaptation and the reversion of antibiotic resistance.
Sandgren, A. et al. Tuberculosis drug resistance mutation database. PLoS Med. 6, e2 (2009).
Orr, H. A. The genetic theory of adaptation: a brief history. Nature Rev. Genet. 6, 119–127 (2005).
Eyre-Walker, A. & Keightley, P. D. The distribution of fitness effects of new mutations. Nature Rev. Genet. 8, 610–618 (2007).
Joyce, P., Rokyta, D. R., Beisel, C. J. & Orr, H. A. A general extreme value theory model for the adaptation of DNA sequences under strong selection and weak mutation. Genetics 180, 1627–1643 (2008).
Orr, H. A. The population genetics of adaptation: the adaptation of DNA sequences. Evolution 56, 1317–1330 (2002). In this landmark theory paper, Orr shows that many very general properties of adaptive walks can be derived from the simple assumption of an exponential DFBM.
Orr, H. A. The distribution of fitness effects among beneficial mutations. Genetics 163, 1519–1526 (2003).
Gillespie, J. H. A simple stochastic gene substitution model. Theor. Popul. Biol. 23, 202–215 (1983).
Kassen, R. & Bataillon, T. Distribution of fitness effects among beneficial mutations before selection in experimental populations of bacteria. Nature Genet. 38, 484–488 (2006).
Sanjuan, R., Moya, A. & Elena, S. F. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl Acad. Sci. USA 101, 8396–8401 (2004).
Baquero, F., Negri, M. C., Morosini, M. L. & Blázquez, J. Selection of very small differences in bacterial evolution. Int. Microbiol. 1, 295–300 (1998).
Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52, 11–17 (2003).
Fischbach, M. A. & Walsh, C. T. Antibiotics for emerging pathogens. Science 325, 1089–1093 (2009).
LeClerc, J. E., Li, B., Payne, W. L. & Cebula, T. A. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274, 1208–1211 (1996).
Matic, I. et al. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science 277, 1833–1834 (1997).
Oliver, A., Cantón, R., Campo, P., Baquero, F. & Blázquez, J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288, 1251–1253 (2000).
Giraud, A., Radman, M., Matic, I. & Taddei, F. The rise and fall of mutator bacteria. Curr. Opin. Microbiol. 4, 582–585 (2001).
Lind, P. A. & Andersson, D. I. Whole-genome mutational biases in bacteria. Proc. Natl Acad. Sci. USA 105, 17878–17883 (2008).
Garibyan, L. et al. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair 2, 593–608 (2003).
Ochman, H., Lawrence, J. G. & Grolsman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Hanage, W. P., Fraser, C., Tang, J., Connor, T. R. & Corander, J. Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science 324, 1454–1457 (2009).
Mazel, D. Integrons: agents of bacterial evolution. Nature Rev. Microbiol. 4, 608–620 (2006).
Martinez, J. L. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc. R. Soc. Lond., B 276, 2521–2530 (2009).
Turner, P. E. Phenotypic plasticity in bacterial plasmids. Genetics 167, 9–20 (2004).
Levin, B. R., Stewart, F. M. & Rice, V. A. The kinetics of conjugative plasmid transmission: fit of a simple mass action model. Plasmid 2, 247–260 (1979).
Dionisio, F., Matic, I., Radman, M., Rodrigues, O. R. & Taddei, F. Plasmids spread very fast in heterogeneous bacterial communities. Genetics 162, 1525–1532 (2002).
Lilley, A. K. & Bailey, M. J. The transfer dynamics of Pseudomonas sp. plasmid pQBR11 in biofilms. FEMS Microbiol. Ecol. 42, 243–250 (2002).
D'Costa, V. M., McGrann, K. M., Hughes, D. W. & Wright, G. D. Sampling the antibiotic resistome. Science 311, 374–377 (2006).
Sommer, M. O., Dantas, G. & Church, G. M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 325, 1128–1131 (2009).
Perron, G. G., Quessy, S. & Bell, G. A reservoir of drug-resistant pathogenic bacteria in asymptomatic hosts. PLoS ONE 3, e3749 (2008).
Burt, A. & Trivers, R. Genes in Conflict (Belknap, Cambridge, Massachusetts, 2006).
Dionisio, F., Conceição, I. C., Marques, A. C. R., Fernandes, L. & Gordo, I. The evolution of a conjugative plasmid and its ability to increase bacterial fitness. Biol. Lett. 1, 250–252 (2005).
Luciani, F., Sisson, S. A., Jiang, H. L., Francis, A. R. & Tanaka, M. M. The epidemiological fitness cost of drug resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 106, 14711–14715 (2009).
Devanas, M. A., Rafaeli-Eshkol, D. & Stotzky, G. Survival of plasmid-containing strains of Escherichia coli in soil: effect of plasmid size and nutrients on survival of hosts and maintenance of plasmids. Curr. Microbiol. 13, 269–277 (1986).
Bouma, J. E. & Lenski, R. E. Evolution of a bacteria/plasmid association. Nature 335, 351–352 (1988).
Dahlberg, C. & Chao, L. Amelioration of the cost of conjugative plasmid carriage in Escherichia coli K12. Genetics 165, 1641–1649 (2003).
Modi, R. I. & Adams, J. Coevolution in bacterial–plasmid populations. Evolution 45, 656–667 (1991).
Enne, V. I., Delsol, A. A., Roe, J. M. & Bennett, P. M. Rifampicin resistance and its fitness cost in Enterococcus faecium. J. Antimicrob. Chemother. 53, 203–207 (2004).
Kugelberg, E., Lofmark, S., Wretlind, B. & Andersson, D. I. Reduction of the fitness burden of quinolone resistance in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 55, 22–30 (2005).
Mariam, D. H., Mengistu, Y., Hoffner, S. E. & Andersson, D. I. Effect of rpoB mutations conferring rifampin resistance on fitness of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48, 1289–1294 (2004).
Trindade, S. et al. Positive epistasis drives the acquisition of multidrug resistance. PLoS Genet. 5, e1000578 (2009). This paper provides the first large-scale test for epistasis between resistance mutations and shows a strong tendency towards antagonistic epistasis between resistance mutations.
Tokuriki, N., Stricher, F., Schymkowitz, J., Serrano, L. & Tawfik, D. S. The stability effects of protein mutations appear to be universally distributed. J. Mol. Biol. 369, 1318–1332 (2007).
Blance, S. J. et al. Temperature-sensitive suppressor mutations of the Escherichia coli DNA gyrase B protein. Protein Sci. 9, 1035–1037 (2000).
Guerois, R., Nielsen, J. E. & Serrano, L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J. Mol. Biol. 320, 369–387 (2002).
Potapov, V., Cohen, M. & Schreiber, G. Assessing computational methods for predicting protein stability upon mutation: good on average but not in the details. Protein Eng. Des. Sel. 22, 553–560 (2009).
Perkins, A. E. & Nicholson, W. L. Uncovering new metabolic capabilities of Bacillus subtilis using phenotype profiling of rifampin-resistant rpoB mutants. J. Bacteriol. 190, 807–814 (2008).
Hall, A. R., Griffiths, V. F., MacLean, R. C. & Colegrave, N. Mutational neighbourhood and mutation supply rate constrain adaptation in Pseudomonas aeruginosa. Proc. R. Soc. Lond., B 277, 643–650 (2010).
Hanson, D. K., Tiede, D. M., Nance, S. L., Chang, C. H. & Schiffer, M. Site-specific and compensatory mutations imply unexpected pathways for proton delivery to the QB binding-site of the photosynthetic reaction-center. Proc. Natl Acad. Sci. USA 90, 8929–8933 (1993).
Reynolds, M. G. Compensatory evolution in rifampin-resistant Escherichia coli. Genetics 156, 1471–1481 (2000).
Björkman, J., Hughes, D. & Andersson, D. I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc. Natl Acad. Sci. USA 95, 3949–3953 (1998).
Maisnier-Patin, S., Paulander, W., Pennhag, A. & Andersson, D. I. Compensatory evolution reveals functional interactions between ribosomal proteins S12 L14 and L19. J. Mol. Biol. 366, 207–215 (2007).
Nilsson, A. I. et al. Reducing the fitness cost of antibiotic resistance by amplification of initiator tRNA genes. Proc. Natl Acad. Sci. USA 103, 6976–6981 (2006).
Romero, D. & Palacios, R. Gene amplification and genomic plasticity in prokaryotes. Annu. Rev. Genet. 31, 91–111 (1997).
Sandegren, L. & Andersson, D. I. Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nature Rev. Microbiol. 7, 578–588 (2009).
Rokyta, D., Badgett, M. R., Molineux, I. J. & Bull, J. J. Experimental genomic evolution: extensive compensation for loss of DNA ligase activity in a virus. Mol. Biol. Evol. 19, 230–238 (2002).
Sherman, D. R. et al. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272, 1641–1643 (1996).
Andersson, D. I. Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 6, 452–456 (2003).
Schrag, S. J., Perrot, V. & Levin, B. R. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. R. Soc. Lond., B 264, 1287–1291 (1997).
Orr, H. A. The population genetics of adaptation: the distribution of factors fixed during adaptive evolution. Evolution 52, 935–949 (1998).
Gillespie, J. H. Molecular evolution over the mutational landscape. Evolution 38, 1116–1129 (1984).
Bjorkman, J., Nagaev, I., Berg, O. G., Hughes, D. & Andersson, D. I. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287, 1479–1482 (2000). In this landmark paper, the authors show that the fitness costs of bacterial resistance and subsequent compensatory adaptation differ between assays done in mice and in culture medium.
Maisnier-Patin, S., Berg, O. G., Liljas, L. & Andersson, D. I. Compensatory adaptation to the deleterious effect of antibiotic resistance in Salmonella typhimurium. Mol. Microbiol. 46, 355–366 (2002).
Moore, F. B. G., Rozen, D. E. & Lenski, R. E. Pervasive compensatory adaptation in Escherichia coli. Proc. R. Soc. Lond., B 267, 515–522 (2000).
Schoustra, S. E., Bataillon, T., Gifford, D. R. & Kassen, R. The properties of adaptive walks in evolving populations of fungus. PLoS Biol. 7, e1000250 (2009).
Paulander, W., Maisnier-Patin, S. & Andersson, D. I. Multiple mechanisms to ameliorate the fitness burden of mupirocin resistance in Salmonella typhimurium. Mol. Microbiol. 64, 1038–1048 (2007).
DePristo, M. A., Weinreich, D. M. & Hartl, D. L. Missense meanderings in sequence space: a biophysical view of protein evolution. Nature Rev. Genet. 6, 678–687 (2005).
Poon, A., Davis, B. H. & Chao, L. The coupon collector and the suppressor mutation: estimating the number of compensatory mutations by maximum likelihood. Genetics 170, 1323–1332 (2005).
Smith, P. A. & Romesberg, F. E. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nature Chem. Biol. 3, 549–556 (2007).
Hauser, A. R. Antibiotics Basics for Clinicians: Choosing the Right Antibacterial Agent, 318 (Lippincott Williams and Wilkins, Baltimore, 2007).
Hegreness, M., Shoresh, N., Damian, D., Hartl, D. & Kishony, R. Accelerated evolution of resistance in multidrug environments. Proc. Natl Acad. Sci. USA 105, 13977–13981 (2008).
Ward, H., Perron, G. & MacLean, R. C. The fitness cost of multiple drug resistance in Pseudomonas aeruginosa. J. Evol. Biol. 22, 997–1003 (2009).
Anderson, J. B., Ricker, N. & Sijursingh, C. Antagonism between two mechanisms of antifungal drug resistance. Eukaryotic Cell 5, 1243–1251 (2006).
Sanjuan, R., Cuevas, J. M., Moya, A. & Elena, S. F. Epistasis and the adaptability of an RNA virus. Genetics 170, 1001–1008 (2005).
Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 8, 1–13 (2008).
Débarre, F., Lenormand, T. & Gandon, S. Evolutionary epidemiology of drug-resistance in space. PLoS Comput. Biol. 5, e1000337 (2009). This theoretical study integrates evolution and epidemiology to investigate how drug resistance evolution is influenced by the spatial context of treatment.
Harris, S. R. et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science 327, 469–474 (2010).
Grundmann, H., Aires- de-Sousa, M., Boyce, J. & Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368, 874–885 (2006).
Séveno, N. A. et al. Occurrence and reservoirs of antibiotic resistance genes in the environment. Rev. Med. Microbiol. 13, 15–27 (2002).
Perron, G. G., Bell, G. & Quessy, S. Parallel evolution of multidrug-resistance in Salmonella enterica isolated from swine. FEMS Microbiol. Lett. 281, 17–22 (2008).
Perron, G. G., Quessy, S., Letellier, A. & Bell, G. Genotypic diversity and antimicrobial resistance in asymptomatic Salmonella enterica serotype Typhimurium DT104. Infect. Genet. Evol. 7, 223–228 (2007).
Sexton, T., Clarke, P., O'Neill, E., Dillane, T. & Humphreys, H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J. Hosp. Infect. 62, 187–194 (2006).
Sánchez-Payá, J. et al. Nosocomial infection surveillance and control: current situation in Spanish hospitals. J. Hosp. Infect. 72, 50–56 (2009).
Seppala, H. et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337, 441–446 (1997).
Pal, C., Macia, M. D., Oliver, A., Schachar, I. & Buckling, A. Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450, 1079–1081 (2007).
Perron, G. G. & Buckling, A. Hypermutability and compensatory adaptation in antibiotic-resistant bacteria. Am. Nat. (in the press).
Bergstrom, C. T., Lo, M. & Lipsitch, M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc. Natl Acad. Sci. USA 101, 13285–13290 (2004).
Smith, D. L., Dushoff, J., Perencevich, E. N., Harris, A. D. & Levin, S. A. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: resistance is a regional problem. Proc. Natl Acad. Sci. USA 101, 3709–3714 (2004).
Martinez, J. L., Baquero, F. & Andersson, D. I. Predicting antibiotic resistance. Nature Rev. Microbiol. 5, 958–965 (2007).
Campbell, E. A. et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104, 901–912 (2001).
Johanson, U., Ævarsson, A., Liljas, A. & Hughes, D. The dynamic structure of EF-G. studied by fusidic acid resistance and internal revertants. J. Mol. Biol. 258, 420–432 (1996).
Madigan, M. T. & Martinko, J. M. Brock Biology of Microorganisms, (Pearson Education, San Francisco, 2006).
Cohan, F. M., King, E. C. & Zawadzki, P. Amelioration of the deleterious pleiotropic effects of an adaptive mutation in Bacillus subtilis. Evolution 48, 81–95 (1994).
Austin, D. J. & Anderson, R. M. Studies of antibiotic resistance within the patient, hospitals and the community using simple mathematical models. Philos. Trans. R. Soc. Lond., B 354, 721–738 (1999).
De Gelder, L. et al. Combining mathematical models and statistical methods to understand and predict the dynamics of antibiotic-sensitive mutants in a population of resistant bacteria during experimental evolution. Genetics 168, 1131–1144 (2004).
Reluga, T. C. Simple models of antibiotic cycling. Math. Med. Biol. 22, 187–208 (2005).
Martin, G., Lenormand, T. & Phillips, P. A general multivariate extension of Fisher's geometric model and the distribution of mutation fitness effects across species. Evolution 60, 893–907 (2009).
Rokyta, D. R. et al. Beneficial fitness effects are not exponential for two viruses. J. Mol. Evol. 67, 368–376 (2008).
Szathmáry, E. Do deleterious mutations interact synergistically? Metabolic control theory provides a partial answer. Genetics 133, 127–132 (1993).
Klipp, E. et al. Systems Biology, (Wiley, Weinheim, Germany, 2009).
Sanjuan, R. & Nebot, M. R. A network model for the correlation between epistasis and genomic complexity. PLoS ONE 3, e2663 (2008).
Smith, E. E. et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl Acad. Sci. 103, 8487–8492 (2006).
Meacci, F. et al. Drug resistance evolution of a Mycobacterium tuberculosis strain from a noncompliant patient. J. Clin. Microbiol. 43, 3114–3120 (2005).
Mwangi, M. M. et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc. Natl Acad. Sci. USA 104, 9451–9456 (2007).
Lipsitch, M., Bergstrom, C. T. & Levin, B. R. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc. Natl Acad. Sci. USA 97, 1938–1943 (2000).
Brown, E. M. & Nathwani, D. Antibiotic cycling or rotation: a systematic review of the evidence of efficacy. J. Antimicrob. Chemother. 55, 6–9 (2005).
Acknowledgements
The authors acknowledge support from the European Research Council, the Royal Society and the Leverhulme Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Strong selection
-
Occurs when selection imposes strong pressure on the evolution of a trait. In the context of this Review, the strength of selection for resistance increases with antibiotic dose. Most experiments select for resistance under antibiotic doses that prevent growth of wild-type strains, implying strong selection for resistance.
- Population bottlenecking
-
Occurs when the size of a population is reduced dramatically.
- Effective population size
-
The effective population size of a population is the number of individuals in a theoretically ideal population that have the same magnitude of genetic drift as the actual population.
- Extreme value theory
-
A branch of statistical theory that is concerned with the asymptotic properties of the largest samples from a probability distribution, that is those from the tail of the distribution.
- Systems-based approach
-
An approach that investigates a biological phenomenon by assaying a wide range of levels of biological organization, from individual proteins to entire cellular networks. In the context of antibiotic resistance, such an approach involves predicting the fitness effects of resistance and compensatory mutations (ideally quantitatively) based on molecular models of resistance and compensation.
- Pleiotropy
-
A phenomenon in which a gene can influence two or more independent characteristics.
- Adaptive walks
-
A metaphor used to describe the sequence of fixation of beneficial mutations that transform a low-fitness genotype into a genotype that is well-adapted to its environment.
- Longitudinal study
-
A study in which repeated measurements are taken from the same subjects at different time points.
Rights and permissions
About this article
Cite this article
MacLean, R., Hall, A., Perron, G. et al. The population genetics of antibiotic resistance: integrating molecular mechanisms and treatment contexts. Nat Rev Genet 11, 405–414 (2010). https://doi.org/10.1038/nrg2778
Issue Date:
DOI: https://doi.org/10.1038/nrg2778
This article is cited by
-
Bottleneck size and selection level reproducibly impact evolution of antibiotic resistance
Nature Ecology & Evolution (2021)
-
Larger bacterial populations evolve heavier fitness trade-offs and undergo greater ecological specialization
Heredity (2020)
-
Bacterial adaptation is constrained in complex communities
Nature Communications (2020)
-
Assessing evolutionary risks of resistance for new antimicrobial therapies
Nature Ecology & Evolution (2019)
-
Identifying and exploiting genes that potentiate the evolution of antibiotic resistance
Nature Ecology & Evolution (2018)