Key Points
-
Rett syndrome is an X-linked dominant neurodevelopmental disorder that affects patients many months after birth, following a period of apparently normal growth and development. It occurs almost exclusively in females, at an incidence of 1/10,000 to 1/15,000 live births.
-
The genetic defects that are responsible for Rett syndrome affect the gene MECP2, which encodes methyl-CpG binding protein 2. Females with Rett syndrome are heterozygous for a de novo mutation in MECP2.
-
Mutations in MECP2, including duplications of the whole gene, are also present at a low frequency in males, and are associated with a range of phenotypes that extend from severe encephalopathy to mild mental retardation.
-
Rett-like syndromes can result from mutations in CDKL5 (cyclin-dependent kinase-like 5), which encodes a serine/threonine kinase that interacts with MeCP2.
-
MeCP2 expression is highly regulated in neurons, and its level increases progressively with neuronal differentiation and maturation.
-
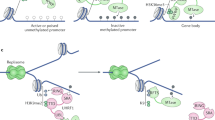
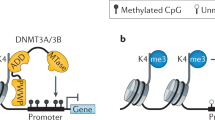
MeCP2 binds methylated DNA and functions as a transcriptional-repressor regulator through a complex that includes the co-repressor SIN3A and histone deacetylases.
-
Knock-out and knock-in Mecp2 mice models, as well as conditional inactivation in mice of MeCP2 in post-mitotic neuronal cells and in postnatal stages, recapitulate symptoms of human Rett syndrome phenotypes.
-
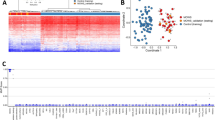
Most transcriptomic studies that have used either animal models or cell lines from patients with Rett syndrome have failed to identify genes that are regulated by MeCP2. However, candidate-gene-based expression studies have contributed into the identification of genes such as BDNF (brain-derived neurotrophic factor), DLX5 (distal-less homeobox 5), UBE3A (ubiquitin protein ligase E3A) and GABRB3 (γ-aminobutyric-acid receptor 3) that are differentially expressed in these systems.
-
The expression of BDNF, a factor that is involved in neuronal morphogenesis and maturation, is deregulated in MeCP2-deficient mice. Intriguingly, BDNF expression is increased at the mRNA level and decreased at the protein level in these mice.
-
In addition to its chromatin-remodelling and transcriptional-regulatory functions, MeCP2 also regulates the expression of genes that are located in imprinted genomic regions and is thought to function as a splicing regulator of neuronal transcripts.
-
MeCP2 does not seem to be a global gene repressor; the evidence that is emerging indicates that Rett syndrome might result from the altered expression of a few key genes that are involved in postnatal, experience-dependent neuronal activity.
Abstract
The discovery that Rett syndrome is caused by mutations that affect the methyl-CpG-binding protein MeCP2 provided a major breakthrough in understanding this severe neurodevelopmental disorder. Animal models and expression studies have contributed to defining the role of MeCP2 in development, highlighting its contribution to postnatal neuronal morphogenesis and function. Furthermore, in vitro assays and microrray studies have delineated the potential molecular mechanisms of MeCP2 function, and have indicated a role in the transcriptional silencing of specific target genes. As well as unravelling the mechanisms that underlie Rett syndrome, these studies provide more general insights into how DNA-methylation patterns are recognized and translated into biological outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Robertson, K. D. DNA methylation and human disease. Nature Rev. Genet. 6, 597–610 (2005). An excellent review of DNA methylation and human disease that documents the links between DNA methylation and cancer, DNA methylation and imprinting disorders, and DNA methylation and repeat-instability diseases.
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185–188 (1999). This is the first report that mutations in MECP2 are responsible for Rett syndrome. Mutations were found in 25% of patients and the authors suggest a loss-of-function mechanism.
Fan, G. & Hutnick, L. Methyl-CpG binding proteins in the nervous system. Cell Res. 15, 255–261 (2005).
Klose, R. J. et al. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell 19, 667–678 (2005).
Nan, X., Campoy, F. J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Jones, P. L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet. 19, 187–191 (1998). References 7 and 8 demonstrated ( in vitro and in cell-culture experiments) that, after binding to methylated CpG dinucleotides, MeCP2 can recruit histone deacetylase to a transcriptional-repressor complex and silence target genes. These studies prove for the first time that MBD-containing proteins such as MeCP2 can function as a molecular link between DNA methylation at promoter regions and transcriptional silencing.
Kokura, K. et al. The Ski protein family is required for MeCP2-mediated transcriptional repression. J. Biol. Chem. 276, 34115–34121 (2001).
Nan, X., Tate, P., Li, E. & Bird, A. DNA methylation specifies chromosomal localization of MeCP2. Mol. Cell. Biol. 16, 414–421 (1996).
Chandler, S. P., Guschin, D., Landsberger, N. & Wolffe, A. P. The methyl-CpG binding transcriptional repressor MeCP2 stably associates with nucleosomal DNA. Biochemistry 38, 7008–7018 (1999).
Buschdorf, J. P. & Stratling, W. H. A WW domain binding region in methyl-CpG-binding protein MeCP2: impact on Rett syndrome. J. Mol. Med. 82, 135–143 (2004).
Kriaucionis, S. & Bird, A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res. 32, 1818–1823 (2004).
Mnatzakanian, G. N. et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nature Genet. 36, 339–341 (2004). References 13 and 14 demonstrate that MeCP2 is subject to alternative splicing — generating two different N termini, one of which is significantly more abundant than the other — especially in the brain.
Amir, R. E. & Zoghbi, H. Y. Rett syndrome: methyl-CpG-binding protein 2 mutations and phenotype-genotype correlations. Am. J. Med. Genet. 97, 147–152 (2000).
Miltenberger-Miltenyi, G. & Laccone, F. Mutations and polymorphisms in the human methyl CpG-binding protein MECP2. Hum. Mutat. 22, 107–115 (2003).
Weaving, L. S., Ellaway, C. J., Gecz, J. & Christodoulou, J. Rett syndrome: clinical review and genetic update. J. Med. Genet. 42, 1–7 (2005).
Philippe, C. et al. Spectrum and distribution of MECP2 mutations in 424 Rett syndrome patients: a molecular update. Eur. J. Med. Genet. 49, 9–18 (2006).
Schollen, E., Smeets, E., Deflem, E., Fryns, J. P. & Matthijs, G. Gross rearrangements in the MECP2 gene in three patients with Rett syndrome: implications for routine diagnosis of Rett syndrome. Hum. Mutat. 22, 116–120 (2003).
Laccone, F. et al. Large deletions of the MECP2 gene detected by gene dosage analysis in patients with Rett syndrome. Hum. Mutat. 23, 234–244 (2004).
Ravn, K. et al. Large genomic rearrangements in MECP2. Hum. Mutat. 25, 324 (2005).
Shi, J. et al. Detection of heterozygous deletions and duplications in the MECP2 gene in Rett syndrome by Robust Dosage PCR (RD-PCR). Hum. Mutat. 25, 505 (2005).
Archer, H. L. et al. Gross rearrangements of the MECP2 gene are found in both classical and atypical Rett Syndrome. J. Med. Genet. 23 Sep 2005 (doi:10.1136/jmg.2005.033464).
Zappella, M., Meloni, I., Longo, I., Hayek, G. & Renieri, A. Preserved speech variants of the Rett syndrome: molecular and clinical analysis. Am. J. Med. Genet. 104, 14–22 (2001).
Charman, T. et al. Dimensional phenotypic analysis and functional categorisation of mutations reveal novel genotype-phenotype associations in Rett syndrome. Eur. J. Hum. Genet. 13, 1121–1130 (2005).
Jian, L. et al. p.R270X MECP2 mutation and mortality in Rett syndrome. Eur. J. Hum. Genet. 13, 1235–1238 (2005).
Ravn, K., Nielsen, J. B., Uldall, P., Hansen, F. J. & Schwartz, M. No correlation between phenotype and genotype in boys with a truncating MECP2 mutation. J. Med. Genet. 40, e5 (2003).
Kleefstra, T. et al. MECP2 analysis in mentally retarded patients: implications for routine DNA diagnostics. Eur. J. Hum. Genet. 12, 24–28 (2004).
Kudo, S. et al. Heterogeneity in residual function of MeCP2 carrying missense mutations in the methyl CpG binding domain. J. Med. Genet. 40, 487–493 (2003).
Masuyama, T. et al. Classic Rett syndrome in a boy with R133C mutation of MECP2. Brain Dev. 27, 439–442 (2005).
Orrico, A. et al. MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett. 481, 285–288 (2000).
Couvert, P. et al. MECP2 is highly mutated in X-linked mental retardation. Hum. Mol. Genet. 10, 941–946 (2001). This paper describes novel mutations in MECP2 that are not found in patients with Rett syndrome but in males with nonspecific mental retardation. Such mutations have been identified in families with recessively inherited mental retardation, and also in sporadic cases of mental retardation in males.
Cohen, D. et al. MECP2 mutation in a boy with language disorder and schizophrenia. Am. J. Psychiatry 159, 148–149 (2002).
Thomas, G. H. High male:female ratio of germ-line mutations: an alternative explanation for postulated gestational lethality in males in X-linked dominant disorders. Am. J. Hum. Genet. 58, 1364–1368 (1996).
Girard, M. et al. Parental origin of de novo MECP2 mutations in Rett syndrome. Eur. J. Hum. Genet. 9, 231–236 (2001).
Trappe, R. et al. MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am. J. Hum. Genet. 68, 1093–1101 (2001).
Yntema, H. G. et al. Low frequency of MECP2 mutations in mentally retarded males. Eur. J. Hum. Genet. 10, 487–490 (2002).
Meins, M. et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J. Med. Genet. 42, e12 (2005).
Van Esch, H. et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 77, 442–453 (2005). The authors demonstrate that, in addition to the defects that are caused by impaired or abolished MECP2 gene function, defects are also caused by increased MeCP2 dosage in humans, which results in a distinct phenotype. Duplications of the MECP2 region occur frequently in male patients with a severe form of mental retardation, which justifies quantitative screening of MECP2 in this group of patients.
Collins, A. L. et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 13, 2679–2689 (2004).
Weaving, L. S. et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am. J. Hum. Genet. 75, 1079–1093 (2004).
Tao, J. et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am. J. Hum. Genet. 75, 1149–1154 (2004).
Mari, F. et al. Germline mosaicism in Rett syndrome identified by prenatal diagnosis. Clin. Genet. 67, 258–260 (2005).
Chen, R. Z., Akbarian, S., Tudor, M. & Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet. 27, 327–331 (2001).
Guy, J., Hendrich, B., Holmes, M., Martin, J. E. & Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genet. 27, 322–326 (2001).
Gemelli, T. et al. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol. Psychiatry 59, 468–476 (2005).
Luikenhuis, S., Giacometti, E., Beard, C. F. & Jaenisch, R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl Acad. Sci. USA 101, 6033–6038 (2004).
Shahbazian, M. et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243–254 (2002).
Young, J. I. & Zoghbi, H. Y. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of Rett syndrome. Am. J. Hum. Genet. 74, 511–520 (2004).
Asaka, Y., Jugloff, D. G., Zhang, L., Eubanks, J. H. & Fitzsimonds, R. M. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 21, 217–227 (2006).
Moretti, P. et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 26, 319–327 (2006).
Shahbazian, M. D., Antalffy, B., Armstrong, D. L. & Zoghbi, H. Y. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 11, 115–124 (2002).
Akbarian, S. The neurobiology of Rett syndrome. Neuroscientist 9, 57–63 (2003).
Balmer, D., Goldstine, J., Rao, Y. M. & LaSalle, J. M. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J. Mol. Med. 81, 61–68 (2003).
Samaco, R. C., Nagarajan, R. P., Braunschweig, D. & LaSalle, J. M. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 13, 629–639 (2004).
LaSalle, J. M., Goldstine, J., Balmer, D. & Greco, C. M. Quantitative localization of heterogeneous methyl-CpG-binding protein 2 (MeCP2) expression phenotypes in normal and Rett syndrome brain by laser scanning cytometry. Hum. Mol. Genet. 10, 1729–1740 (2001).
Armstrong, D., Dunn, J. K., Antalffy, B. & Trivedi, R. Selective dendritic alterations in the cortex of Rett syndrome. J. Neuropathol. Exp. Neurol. 54, 195–201 (1995).
Armstrong, D. D., Dunn, K. & Antalffy, B. Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. J. Neuropathol. Exp. Neurol. 57, 1013–1017 (1998).
Kishi, N. & Macklis, J. D. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci 27, 306–321 (2004).
Cusack, S. M. et al. Suppression of MeCP2β expression inhibits neurite extension in PC12 cells. Exp. Cell Res. 299, 442–453 (2004).
Kaufmann, W. E., Naidu, S. & Budden, S. Abnormal expression of microtubule-associated protein 2 (MAP-2) in neocortex in Rett syndrome. Neuropediatrics 26, 109–113 (1995).
Johnston, M. V., Jeon, O. H., Pevsner, J., Blue, M. E. & Naidu, S. Neurobiology of Rett syndrome: a genetic disorder of synapse development. Brain Dev. 23, S206–S213 (2001).
Colantuoni, C. et al. Gene expression profiling in postmortem Rett Syndrome brain: differential gene expression and patient classification. Neurobiol. Dis. 8, 847–865 (2001).
Pescucci, C. et al. Chromosome 2 deletion encompassing the MAP2 gene in a patient with autism and Rett-like features. Clin. Genet. 64, 497–501 (2003).
Nan, X., Cross, S. & Bird, A. Gene silencing by methyl-CpG-binding proteins. Novartis Found. Symp. 214, 6–50 (1998).
Wan, M., Zhao, K., Lee, S. S. & Francke, U. MECP2 truncating mutations cause histone H4 hyperacetylation in Rett syndrome. Hum. Mol. Genet. 10, 1085–1092 (2001).
Kaufmann, W. E. et al. Histone modifications in Rett syndrome lymphocytes: a preliminary evaluation. Brain Dev. 27, 331–339 (2005).
Kouzarides, T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12, 198–209 (2002).
Fuks, F. et al. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 278, 4035–4040 (2003).
Fuks, F., Hurd, P. J., Deplus, R. & Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 31, 2305–2312 (2003).
Georgel, P. T. et al. Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation. J. Biol. Chem. 278, 32181–32188 (2003).
Traynor, J., Agarwal, P., Lazzeroni, L. & Francke, U. Gene expression patterns vary in clonal cell cultures from Rett syndrome females with eight different MECP2 mutations. BMC Med. Genet. 3, 12 (2002).
Tudor, M., Akbarian, S., Chen, R. Z. & Jaenisch, R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc. Natl Acad. Sci. USA 99, 15536–15541 (2002).
Ballestar, E. et al. The impact of MECP2 mutations in the expression patterns of Rett syndrome patients. Hum. Genet. 116, 91–104 (2005).
Matarazzo, V. & Ronnett, G. V. Temporal and regional differences in the olfactory proteome as a consequence of MeCP2 deficiency. Proc. Natl Acad. Sci. USA 101, 7763–7768 (2004).
Nuber, U. A. et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum. Mol. Genet. 14, 2247–2256 (2005).
Martinowich, K. et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302, 890–893 (2003).
Chen, W. G. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 (2003). References 77 and 78 show that MeCP2 binds selectively to the BDNF promoter and represses the dynamic expression of BDNF , which is regulated by neuronal activity. These reports indicate that the deregulation of activity-dependent transcription that occurs when MECP2 is mutated could affect synaptic development and contribute to the pathology of Rett syndrome.
Webster, M. J., Weickert, C. S., Herman, M. M. & Kleinman, J. E. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res. Dev. Brain Res. 139, 139–150 (2002).
Scharfman, H. E. Brain-derived neurotrophic factor and epilepsy — a missing link? Epilepsy Curr. 5, 83–88 (2005).
Yamada, K. & Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 91, 267–270 (2003).
Croll, S. D. et al. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience 93, 1491–1506 (1999).
Richerson, G. B. & Bekkers, J. M. Learning to take a deep breath — with BDNF. Nature Med. 10, 25–26 (2004).
Gorski, J. A., Zeiler, S. R., Tamowski, S. & Jones, K. R. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J. Neurosci. 23, 6856–6865 (2003).
Gorski, J. A., Balogh, S. A., Wehner, J. M. & Jones, K. R. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 121, 341–354 (2003).
Chang, Q., Khare, G., Dani, V., Nelson, S. & Jaenisch, R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49, 341–348 (2006). The authors provide the first in vivo evidence for a functional interaction between MeCP2 and BDNF in mice and demonstrate that brain BDNF levels can modulate Rett disease progression.
Makedonski, K., Abuhatzira, L., Kaufman, Y., Razin, A. & Shemer, R. MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression. Hum. Mol. Genet. 14, 1049–1058 (2005). The authors describe epigenetic aberrations at the Prader Willi syndrome–Angelman syndrome imprinting centre in patients with Rett and MeCP2-deficient mice. These changes result in loss of imprinting of the UBE3A antisense gene in the brain, an increase in UBE3A antisense RNA levels and, consequently, reduction in UBE3A protein production.
Horike, S., Cai, S., Miyano, M., Cheng, J. F. & Kohwi-Shigematsu, T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nature Genet. 37, 31–40 (2005).
Samaco, R. C., Hogart, A. & LaSalle, J. M. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum. Mol. Genet. 14, 483–492 (2005).
Williams, C. A. Neurological aspects of the Angelman syndrome. Brain Dev. 27, 88–94 (2005).
Young, J. I. et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl Acad. Sci. USA 102, 17551–1758 (2005). This is the first study to describe a function of MeCP2 in the regulation of splicing, in addition to its role as a transcriptional repressor.
Mu, Y., Otsuka, T., Horton, A. C., Scott, D. B. & Ehlers, M. D. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40, 581–594 (2003).
Stancheva, I., Collins, A. L., Van den Veyver, I. B., Zoghbi, H. & Meehan, R. R. A mutant form of MeCP2 protein associated with human Rett syndrome cannot be displaced from methylated DNA by notch in Xenopus embryos. Mol. Cell 12, 425–435 (2003).
Kornblihtt, A. R., de la Mata, M., Fededa, J. P., Munoz, M. J. & Nogues, G. Multiple links between transcription and splicing. RNA 10, 1489–1498 (2004).
Acknowledgements
We apologize to those whose work could not be cited owing to space limitations. We are grateful to the French Association for Rett Syndrome (AFSR). We would like to thank F. Francis for helpful comments. Work in the authors' laboratory is supported by research grants from the European Commission, GIS-Maladies Rares and Fondation France Telecom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Encephalopathy
-
A degenerative condition of the brain that can be caused by infectious disease, metabolic abnormalities, brain tumours, toxic drug effects or increased intercranial pressure.
- Array CGH
-
A technique that uses comparative genomic hybridization on microarrays to determine copy-number differences between DNA sequences.
- Nestin
-
A class VI intermediate-filament protein that is expressed during early embryonic development in mammals but is absent from nearly all mature CNS cells.
- Basal ganglia
-
Clusters of neurons that are located deep in the brain that relay messages between the most anterior part of the cortex that is involved in problem solving and complex thought, and the lower motor and sensory areas; includes the striatum.
- Kyphosis
-
Abnormal curvature of the thoracic spine.
- Astrocyte
-
A star-shaped neuroglial cell that surrounds and supports neurons in the CNS.
- Oligodendrocyte
-
A type of non-neuronal cell that lacks axons and dendrites and insulates axons so that they can send electric impulses. These are also myelin-producing cells.
- Dendritic arborization
-
The process that leads to the production of the numerous dendrites that characterize many neurons, and the branches that project from these dendrites.
- Dendritic spine
-
Mushroom-shaped structures on neuronal dendrites that receive synaptic input and are the sites of postsynaptic densities. Changes in spine shape are thought to be important for modulating synaptic strength.
- Morpholinos
-
Oligonucleotides that are efficient gene-silencing reagents, and that can either block translation initiation in the cytosol or modify pre-mRNA splicing in the nucleus (by targeting splice junctions).
- PC12 cells
-
A clonal cell line that responds reversibly to nerve growth factor.
- Prader-Willi syndrome
-
A genetic disorder that is caused by loss of paternally expressed genes that are located in the 4-Mb imprinted region of 15q11–q13. Features of the disorder include excessive eating (hyperphagia), obesity, short stature, mental retardation or learning disabilities, and behavioural problems.
- Angelman syndrome
-
A genetic disorder that is caused by several genetic mechanisms that inactivate or disrupt the maternally derived UBE3A. Symptoms include hyperactivity, ataxia, problems with speech and language, and an unusually happy demeanour.
- Ubiquitin ligase
-
A protein that is involved in polyubiquitylation that covalently attaches ubiquitin to a lysine residue on a target protein. Polyubiquitylation marks proteins for degradation by the proteasome.
- γ-Aminobutyric acid
-
A major inhibitory neurotransmitter in the mammalian CNS that participates in the regulation of neuronal excitability through interaction with specific receptors.
Rights and permissions
About this article
Cite this article
Bienvenu, T., Chelly, J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat Rev Genet 7, 415–426 (2006). https://doi.org/10.1038/nrg1878
Issue Date:
DOI: https://doi.org/10.1038/nrg1878
This article is cited by
-
Paediatric syndromic scoliosis: proceedings of the half-day course at the 57th annual meeting of the Scoliosis Research Society
Spine Deformity (2024)
-
KDM6B cooperates with Tau and regulates synaptic plasticity and cognition via inducing VGLUT1/2
Molecular Psychiatry (2022)
-
High rate of HDR in gene editing of p.(Thr158Met) MECP2 mutational hotspot
European Journal of Human Genetics (2020)
-
FOXG1 Regulates PRKAR2B Transcriptionally and Posttranscriptionally via miR200 in the Adult Hippocampus
Molecular Neurobiology (2019)
-
Neural stem cells from a mouse model of Rett syndrome are prone to senescence, show reduced capacity to cope with genotoxic stress, and are impaired in the differentiation process
Experimental & Molecular Medicine (2018)