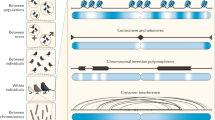
Key Points
-
One effect of recombination is to determine the extent of linkage disequilibrium in population DNA samples.
-
Direct measurement of the recombination rate is difficult and often impractical. For this reason, population-genetic methods are often used to infer recombination rates from patterns of variation among DNA sequences.
-
Population-genetic methods can detect variation in the recombination rate at the level of single genes.
-
Although simple parsimony methods allow the number of recombination events to be counted, most recombination events are missed using this approach.
-
Sophisticated statistical approaches use population-genetic models to estimate recombination rates.
-
Several statistical methods that estimate the population recombination rate have been developed. These are influenced by population history, but can provide important insights into details of the recombination process.
-
Biologically important inferences can be drawn from these estimators even if the underlying assumptions are oversimplified.
-
Discrepancies between estimated and experimentally measured rates can reveal important biological processes.
-
Estimated recombination rates enable the detailed interpretation of linkage disequilibrium and haplotype data.
Abstract
Obtaining an accurate measure of how recombination rates vary across the genome has implications for understanding the molecular basis of recombination, its evolutionary significance and the distribution of linkage disequilibrium in natural populations. Although measuring the recombination rate is experimentally challenging, good estimates can be obtained by applying population-genetic methods to DNA sequences taken from natural populations. Statistical methods are now providing insights into the nature and scale of variation in the recombination rate, particularly in humans. Such knowledge will become increasingly important owing to the growing use of population-genetic methods in biomedical research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hartl, D. L. & Clark, A. G. Principles of Population Genetics (Sinauer, Sunderland, 1998).
Weiss, K. M. & Clark, A. G. Linkage disequilibrium and the mapping of complex human traits. Trends Genet. 18, 19–24 (2002). This work highlights issues that are related to the application of LD data to association studies.
Kaplan, N. & Morris, R. Prospects for association-based fine mapping of a susceptibility gene for a complex disease. Theor. Popul. Biol. 60, 181–191 (2001).
Jeffreys, A. J., Ritchie, A. & Neumann, R. High resolution analysis of haplotype diversity and meiotic crossover in the human TAP2 recombination hotspot. Hum. Mol. Genet. 9, 725–733 (2000).
Badge, R. M., Yardley, J., Jeffreys, A. J. & Armour, J. A. Crossover breakpoint mapping identifies a subtelomeric hotspot for male meiotic recombination. Hum. Mol. Genet. 9, 1239–1244 (2000).
Cullen, M., Erlich, H., Klitz, W. & Carrington, M. Molecular mapping of a recombination hotspot located in the second intron of the human TAP2 locus. Am. J. Hum. Genet. 56, 1350–1358 (1995).
Zhao, H. Family-based association studies. Stat. Methods Med. Res. 9, 563–87 (2000).
Cardon, L. R. & Bell, J. I. Association study designs for complex diseases. Nature Rev. Genet. 2, 91–99 (2001).
Jeffreys, A. J., Murray, J. & Neumann, R. High-resolution mapping of crossovers in human sperm defines a minisatellite-associated recombination hotspot. Mol. Cell 2, 267–273 (1998).
Fearnhead, P. & Donnelly, P. Estimating recombination rates from population genetic data. Genetics 159, 1299–1318 (2001).
Fearnhead, P. & Donnelly, P. Approximate likelihood methods for estimating local recombination rates. J. R. Stat. Soc. Ser. B Stat. Methodol. 64, 657–680 (2002).
Kuhner, M. K., Yamato, J. & Felsenstein, J. Maximum likelihood estimation of recombination rates from population data. Genetics 156, 1393–1401 (2000).
Stephens, M. & Donnelly, P. Inference in molecular population genetics. J. R. Stat. Soc. Ser. B Stat. Methodol. 62, 605–635 (2000).
Pritchard, J. K. & Przeworski, M. Linkage disequilibrium in humans: models and data. Am. J. Hum. Genet. 69, 1–14 (2001). A comprehensive review of LD and its dependence on demography; the paper also examines the connection between theoretical models and experimental data.
Golding, G. B. The sampling distribution of linkage disequilibrium. Genetics 108, 257–274 (1984).
Kruglyak, L. Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nature Genet. 22, 139–144 (1999).
Calafell, F., Grigorenko, E. L., Chikanian, A. A. & Kidd, K. K. Haplotype evolution and linkage disequilibrium: a simulation study. Hum. Hered. 51, 85–96 (2000).
Wang, N., Akey, J. M., Zhang, K., Chakraborty, R. & Jin, L. Distribution of recombination crossovers and the origin of haplotype blocks: the interplay of population history, recombination, and mutation. Am. J. Hum. Genet. 71, 1227–1234 (2002).
Barton, N. H. Genetic hitchhiking. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 355, 1553–1562 (2000).
Charlesworth, B., Nordborg, M. & Charlesworth, D. The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided populations. Genet. Res. 70, 155–174 (1997).
Chapman, N. H. & Thompson, E. A. Linkage disequilibrium mapping: the role of population history, size, and structure. Adv. Genet. 42, 413–437 (2001).
Freimer, N. B., Service, S. K. & Slatkin, M. Expanding on population studies. Nature Genet. 17, 371–373 (1997).
Hudson, R. R. The sampling distribution of linkage disequilibrium under an infinite allele model without selection. Genetics 109, 611–631 (1985).
Garner, C. & Slatkin, M. On selecting markers for association studies: patterns of linkage disequilibrium between two and three diallelic loci. Genet. Epidemiol 24, 57–67 (2003).
Phillips, M. S. et al. Chromosome-wide distribution of haplotype blocks and the role of recombination hot spots. Nature Genet. 33, 382–387 (2003). A study of a dense marker map on chromosome 19 that, together with a detailed theoretical analysis, highlights problems in defining haplotype blocks.
Cardon, L. R. & Abecasis, G. R. Using haplotype blocks to map human complex trait loci. Trends Genet. 19, 135–140 (2003).
Akey, J. M., Zhang, K., Xiong, M. M. & Jin, L. The effect of single nucleotide polymorphism identification strategies on estimates of linkage disequilibrium. Mol. Biol. Evol. 20, 232–242 (2003).
Nielsen, R. & Signorovitch, J. Correcting for ascertainment bias when analyzing SNP data: applications to the estimation of linkage disequilibrium. Theor. Popul. Biol. 63, 245–255 (2003).
Rannala, B. & Slatkin, M. Likelihood analysis of disequilibrium mapping, and related problems. Am. J. Hum. Genet. 62, 459–473 (1998).
Zollner, S. & von Haeseler, A. A coalescent approach to study linkage disequilibrium between single-nucleotide polymorphisms. Am. J. Hum. Genet. 66, 615–628 (2000).
Nordborg, M. & Tavare, S. Linkage disequilibrium: what history has to tell us. Trends Genet. 18, 83–90 (2002). A careful attempt at discussing the effects of population history on LD in a genealogical framework.
Stumpf, M. P. H. & Goldstein, D. B. Genealogical and evolutionary inference with the human Y chromosome. Science 291, 1738–1742 (2001).
Donnelly, P. & Tavare, S. Coalescents and genealogical structure under neutrality. Annu. Rev. Genet. 29, 401–421 (1995).
Nordborg, M. in Handbook of Statistical Genetics (eds Balding, D. J. M. B. & Cannings, C.) 179–212 (Wiley, Chichester, 2000). A modern exposition of the coalescent and its application in modern population genetics.
Hudson, R. R. in Oxford Surveys in Evolutionary Biology (ed. Futuyama, D. J. A.) 1–43 (Oxford University Press, Oxford, 1990).
Tavare, S. A genealogical view of some stochastic-models in population-genetics. Stochastic Processes and their Applications Abstr. 19, 10 (1985).
Tavare, S., Balding, D. J., Griffiths, R. C. & Donnelly, P. Inferring coalescence times from DNA sequence data. Genetics 145, 505–518 (1997).
Stephens, M. in Handbook of Statistical Genetics (eds Balding, D. J. M. B. & Cannings, C.) 213–238 (Wiley, Chichester, 2001). A detailed and highly accessible account of statistical inference in population genetics using the coalescent.
Griffiths, R. C. & Marjoram, P. Ancestral inference from samples of DNA sequences with recombination. J. Comput. Biol. 3, 479–502 (1996).
Hudson, R. R. & Kaplan, N. L. The coalescent process in models with selection and recombination. Genetics 120, 831–840 (1988).
Wiuf, C. & Hein, J. The ancestry of a sample of sequences subject to recombination. Genetics 151, 1217–1228 (1999).
Wiuf, C. & Hein, J. Recombination as a point process along sequences. Theor. Popul. Biol. 55, 248–259 (1999).
Kuhner, M. K., Beerli, P., Yamato, J. & Felsenstein, J. Usefulness of single nucleotide polymorphism data for estimating population parameters. Genetics 156, 439–447 (2000).
Weir, B. S. Inferences about linkage disequilibrium. Biometrics 35, 235–254 (1979).
Myers, S. R. & Griffiths, R. C. Bounds on the minimum number of recombination events in a sample history. Genetics 163, 375–394 (2003).
Wiuf, C. On the minimum number of topologies explaining a sample of DNA sequences. Theor. Popul. Biol. 62, 357–363 (2002).
Posada, D. & Crandall, K. A. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc. Natl Acad. Sci. USA 98, 13757–13762 (2001).
Wiuf, C., Christensen, T. & Hein, J. A simulation study of the reliability of recombination detection methods. Mol. Biol. Evol. 18, 1929–1939 (2001).
McVean, G. A. A genealogical interpretation of linkage disequilibrium. Genetics 162, 987–991 (2002). This paper discusses LD in a genealogical framework and shows how features of the genealogy are connected to LD summary statistics.
Myers, S. The Detection of Recombination Events Using DNA Sequence Data. Thesis, Univ. Oxford (2003).
Wiuf, C. & Hein, J. On the number of ancestors to a DNA sequence. Genetics 147, 1459–1468 (1997).
Kingman, J. F. C. The coalescent. Stochastic Processes and their Applications 13, 235–248 (1982).
Rosenberg, N. A. & Nordborg, M. Genealogical trees, coalescent theory and the analysis of genetic polymorphisms. Nature Rev. Genet. 3, 380–390 (2002).
Wiuf, C. & Posada, D. A coalescent model of recombination hotspots. Genetics 164, 407–417 (2003).
Cavalli-Sforza, L. L., Mennazzi, P. & Piazza, A. The History and Geography of Human Genes (Princeton Univ. Press, Princeton, 1996).
Rannala, B. Gene genealogy in a population of variable size. Heredity 78, 417–423 (1997).
Wakeley, J. & Lessard, S. Theory of the effects of population structure and sampling on patterns of linkage disequilibrium applied to genomic data from humans. Genetics 164, 1043–1053 (2003).
Nordborg, M. Linkage disequilibrium, gene trees and selfing: an ancestral recombination graph with selfing. Genetics 154, 923–929 (2000).
Hey, J. & Wakeley, J. A coalescent estimator of the population recombination rate. Genetics 145, 833–846 (1997).
Wall, J. D. A comparison of estimators of the population recombination rate. Mol. Biol. Evol. 17, 156–163 (2000).
Cox, D. R. & Hinkley, D. V. Theoretical Statistics (Chapman and Hall, London, 1974).
Casella, G. & Berger, R. L. Statistical Inference (Duxbury, Pacific Grove, 2002).
Steel, M. & Penny, D. Parsimony, likelihood, and the role of models in molecular phylogenetics. Mol. Biol. Evol. 17, 839–850 (2000).
Reich, D. E. et al. Linkage disequilibrium in the human genome. Nature 411, 199–204 (2001).
Gabriel, S. B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002). An influential experimental study that investigates the presence of haplotype blocks in different populations across 52 genomic regions.
Jeffreys, A. J., Kauppi, L. & Neumann, R. Intensely punctate meiotic recombination in the class II region of the major histocompatibility complex. Nature Genet. 29, 217–222 (2001). A beautiful experimental study of recombination hotspots and associated patterns of LD in a human population sample.
Clark, A. G. et al. Haplotype structure and population genetic inferences from nucleotide-sequence variation in human lipoprotein lipase. Am. J. Hum. Genet. 63, 595–612 (1998).
Hudson, R. R. Two-locus sampling distributions and their application. Genetics 159, 1805–1817 (2001). The first study to estimate recombination rates using pairwise approximation to the likelihood.
McVean, G., Awadalla, P. & Fearnhead, P. A coalescent-based method for detecting and estimating recombination from gene sequences. Genetics 160, 1231–1241 (2002).
Li, N. & Stephens, M. A new multilocus model for linkage disequilibrium, with application to exploring variations in recombination rate. Genetics (in the press).
Fearnhead, P. Consistency of estimators of the population-scaled recombination rate. Theor. Popul. Biol. 64, 67–79 (2003).
Ardlie, K. G., Kruglyak, L. & Seielstad, M. Patterns of linkage disequilibrium in the human genome. Nature Rev. Genet. 3, 299–309 (2002).
Stumpf, M. P. & Goldstein, D. B. Demography, recombination hotspot intensity, and the block structure of linkage disequilibrium. Curr. Biol. 13, 1–8 (2003).
Stumpf, M. P. Haplotype diversity and the block structure of linkage disequilibrium. Trends Genet. 18, 226–228 (2002).
Reich, D. E. et al. Human genome sequence variation and the influence of gene history, mutation and recombination. Nature Genet. 32, 135–142 (2002).
Frisse, L. et al. Gene conversion and different population histories may explain the contrast between polymorphism and linkage disequilibrium levels. Am. J. Hum. Genet. 69, 831–843 (2001).
Sabeti, P. C. et al. Detecting recent positive selection in the human genome from haplotype structure. Nature 419, 832–837 (2002).
Przeworski, M. & Wall, J. D. Why is there so little intragenic linkage disequilibrium in humans? Genet. Res. 77, 143–151 (2001).
Griffiths, R. C. & Tavare, S. Ancestral inference in population-genetics. Stat. Sci. 9, 307–319 (1994).
Smith, J. M., Smith, N. H., O'Rourke, M. & Spratt, B. G. How clonal are bacteria? Proc. Natl Acad. Sci. USA 90, 4384–4388 (1993).
Smith, J. M. The detection and measurement of recombination from sequence data. Genetics 153, 1021–1027 (1999).
Holmes, E. C. On the origin and evolution of the human immunodeficiency virus (HIV). Biol. Rev 76, 239–254 (2001).
Fu, Y. X. Estimating mutation rate and generation time from longitudinal samples of DNA sequences. Mol. Biol. Evol. 18, 620–626 (2001).
Awadalla, P. The evolutionary genomics of pathogen recombination. Nature Rev. Genet. 4, 50–60 (2003).
Drummond, A. J., Nicholls, G. K., Rodrigo, A. G. & Solomon, W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics 161, 1307–1320 (2002).
Grassly, N. C. & Holmes, E. C. A likelihood method for the detection of selection and recombination using nucleotide sequences. Mol. Biol. Evol. 14, 239–247 (1997).
Hey, J. & Harris, E. Population bottlenecks and patterns of human polymorphism. Mol. Biol. Evol. 16, 1423–1426 (1999).
Nordborg, M. & Donnelly, P. The coalescent process with selfing. Genetics 146, 1185–1195 (1997).
Przeworski, M. The signature of positive selection at randomly chosen loci. Genetics 160, 1179–1189 (2002).
Posada, D. & Wiuf, C. Simulating haplotype blocks in the human genome. Bioinformatics 19, 289–290 (2003).
Gillespie, J. H. Population Genetics: a Concise Guide (Johns Hopkins Univ. Press, Baltimore, 1998).
Wall, J. D. Recombination and the power of statistical tests of neutrality. Genet. Res. 74, 65–79 (1999).
Brown, C. J., Garner, E. C., Dunker, A. K. & Joyce, P. The power to detect recombination using the coalescent. Mol. Biol. Evol. 18, 1421–1424 (2001).
Gillespie, J. H. The Causes of Molecular Evolution (Oxford Univ. Press, Oxford, 1991).
Przeworski, M., Charlesworth, B. & Wall, J. D. Genealogies and weak purifying selection. Mol. Biol. Evol. 16, 246–252 (1999).
Johnson, G. C. et al. Haplotype tagging for the identification of common disease genes. Nature Genet. 29, 233–237 (2001). This paper pioneered the concept of haplotype tagging to describe genetic variation.
Wall, J. D. & Pritchard, J. K. Assessing the performance of haplotype block models of linkage disequilibrium. Am. J. Hum. Genet. 73, 502–515 (2003).
Wall, J. D. & Pritchard, J. K. Haplotype blocks and linkage disequilibrium in the human genome. Nature Rev. Genet. 4, 587–597 (2003).
Anderson, E. C. & Novembre, J. Finding haplotype block boundaries by using the minimum-description-length principle. Am. J. Hum. Genet. 73, 336–354 (2003).
Koivisto, M. et al. in Pac. Symp. Biocomput. 2003 (eds Altman, R. B., Dukner, A. K., Hunter, L., Jung, T. A. & Klein, T. E.) 502–513 (World Scientific, Singapore, 2002).
Liu, J. S. Monte Carlo Strategies in Scientific Computing (Springer, New York, 2003).
Nielsen, R. Estimation of population parameters and recombination rates from single nucleotide polymorphisms. Genetics 154, 931–942 (2000).
Stephens, M., Smith, N. J. & Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68, 978–989 (2001).
Watterson, G. A. On the number of segregating sites in genetic models without recombination. Theor. Popul. Biol. 7, 256–276 (1975).
Acknowledgements
We thank A. Jeffreys and P. Donnelly for useful discussions, and C. Wiuf, M. Slatkin, L. Cardon, G. Coop, C. Spencer and three anonymous referees for their helpful comments on earlier drafts of this manuscript. Generous support through research fellowships from the Wellcome Trust (to M.P.H.S) and the Royal Society (to G.A.T.M.) is gratefully acknowledged.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Glossary
- LINKAGE DISEQUILIBRIUM
-
(LD). A measure of genetic associations between alleles at different loci, which indicates whether allelic or marker associations on the same chromosome are more common than expected.
- MARGINAL GENEALOGY
-
The part of a genealogical graph that corresponds to a single locus or stretch of DNA that is inherited without recombination.
- MARKER ASCERTAINMENT
-
The process by which new genetic markers are obtained — for example, by re-sequencing a subset of chromosomes in a population sample. If those markers are population-specific then inferences that are based on them in other populations might be biased through so-called ascertainment bias.
- HAPLOTYPE
-
The combination of alleles or genetic markers that is found on a single chromosome of a given individual.
- INFINITE SITES MUTATION MODEL
-
A model that assumes that there are an infinite number of nucleotide sites and consequently that each new mutation occurs at a different locus.
- FOUR-GAMETE TEST
-
(FGT). If all four possible gametes are observed for two bi-allelic loci then this test infers that a recombination event must have occurred between them (under an infinite sites mutation model).
- PER-GENERATION RECOMBINATION RATE
-
(r). The probability of a recombination event occurring during meiosis.
- EFFECTIVE POPULATION SIZE
-
(Ne). The size of the ideal constant-size population, in which the effects of random drift would be the same as those seen in the actual population.
- POPULATION RECOMBINATION RATE
-
(ρ). Population-genetic parameters are generally proportional to the product of a molecular per-generation rate (for example, the per-generation recombination rate, r) and the effective population size (Ne). The population recombination rate has therefore often been defined as ρ = 4Ner.
- CENSUS POPULATION SIZE
-
Actual population size (total number of individuals) as compared to the theoretical effective population size.
- ESTIMATOR
-
A statistical method that is used to obtain a numerical estimate for a quantity of interest, such as a model parameter.
- SUMMARY STATISTIC
-
A statistical function that summarizes complex data in terms of simple numbers (examples include the mean and variance).
- VARIANCE
-
A statistic that quantifies the dispersion of data about the mean.
- LIKELIHOOD SURFACE
-
The likelihood of a parameter is proportional to the probability of obtaining the observed data under a parametric model given the model parameter. The likelihood surface is a function/curve that specifies how well the data agrees with the predictions made by a parametric model for different values of the model parameter.
- MARKOV CHAIN MONTE CARLO
-
A computational technique for the efficient numerical calculation of likelihoods.
- RECURSION
-
A repeated mathematical operation that is often used to aid numerical analysis.
- GENE CONVERSION
-
The non-reciprocal transfer of genetic information between homologous genes as a consequence of mismatch repair after heteroduplex formation.
- PHASING
-
Determining the haplotype phase (the arrangement of alleles at two loci on homologous chromosomes) from genotype data using statistical methods.
- ASSOCIATION STUDIES
-
A set of methods that are used to correlate polymorphisms in genotype to polymorphisms in phenotype in populations.
- MODEL MIS-SPECIFICATION
-
The consequence of using a parametric model in the inference process that is different from the true model under which the data was generated.
- CPG ISLANDS
-
Genome sequences of >200 base pairs that have high G+C content and CpG frequency.
- TEMPLATE SWITCHING
-
The process by which RNA templates are switched between viral genomes during reverse transcription.
- BOTTLENECK
-
A temporary marked reduction in population size.
- SELECTIVE SWEEP
-
The process by which positive selection for a mutation eliminates neutral variation at linked sites.
- HARDY–WEINBERG EQUILIBRIUM
-
A state in which the frequency of each diploid genotype at a locus equals that expected from the random union of alleles.
- HAPLOTYPE-BASED APPROACH
-
An approach to association studies in which the co-inheritance of phenotypes and haplotypes — as opposed to single markers — is statistically analysed.
- TAGGING APPROACH
-
Identifying sub-sets of markers ('tags') that describe patterns of association or haplotypes among larger marker sets.
- MINIMUM-DESCRIPTION LENGTH APPROACHES
-
A concept from information theory, in which all of the information contained in a system (for example, a sample of DNA sequences) is described in the most compact form possible.
Rights and permissions
About this article
Cite this article
Stumpf, M., McVean, G. Estimating recombination rates from population-genetic data. Nat Rev Genet 4, 959–968 (2003). https://doi.org/10.1038/nrg1227
Issue Date:
DOI: https://doi.org/10.1038/nrg1227
This article is cited by
-
Consequences of Genetic Recombination on Protein Folding Stability
Journal of Molecular Evolution (2023)
-
Genome-wide recombination map construction from single sperm sequencing in cattle
BMC Genomics (2022)
-
BREC: an R package/Shiny app for automatically identifying heterochromatin boundaries and estimating local recombination rates along chromosomes
BMC Bioinformatics (2021)
-
From molecules to populations: appreciating and estimating recombination rate variation
Nature Reviews Genetics (2020)
-
Contrasting patterns of nucleotide polymorphism suggest different selective regimes within different parts of the PgiC1 gene in Festuca ovina L.
Hereditas (2017)