Key Points
-
The use of plants as expression hosts for the large-scale production of recombinant proteins is a recent innovation that has potential advantages of economy, scalability and safety over traditional expression systems.
-
Many pharmaceutical proteins have been expressed in plants as part of 'proof of principle' studies, including human and animal proteins, recombinant subunit vaccines and recombinant antibodies. Only a few of these proteins have reached advanced stages of development and even fewer have begun clinical trials.
-
Most pharmaceutical proteins have been produced in transgenic tobacco plants, because tobacco has a long history as a model organism and robust expression constructs are available. However, there is increasing interest in the use of other species, particularly cereals, legumes, fruit and vegetables.
-
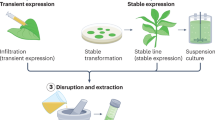
Diverse plant-based expression systems, such as transient expression, plant cell-suspension cultures, recombinant plant viruses and the chloroplast transgenic system are being investigated.
-
Pharmaceutical proteins that are expressed in dry cereal and legume seeds are highly stable and can be stored for long periods at room temperature with no loss of activity.
-
The expression of antibodies and vaccines in edible fruit and vegetables might allow oral administration in partially processed plant tissues.
-
There are some differences between the glycan structures of recombinant glycoproteins that are produced in animals and plants, but so far there is no evidence that such differences cause adverse reactions in human patients.
-
The acceptability of plant-derived pharmaceutical proteins depends on the production of such proteins under cGMP conditions, in line with other expression systems.
Abstract
Imagine a world in which any protein, either naturally occurring or designed by man, could be produced safely, inexpensively and in almost unlimited quantities using only simple nutrients, water and sunlight. This could one day become reality as we learn to harness the power of plants for the production of recombinant proteins on an agricultural scale. Molecular farming in plants has already proven to be a successful way of producing a range of technical proteins. The first plant-derived recombinant pharmaceutical proteins are now approaching commercial approval, and many more are expected to follow.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Schwartz, J. R. Advances in Escherichia coli production of therapeutic proteins. Curr. Opin. Biotechnol. 12, 195–201 (2001).
Chu, L. & Robinson, D. K. Industrial choices for protein production by large-scale cell culture. Curr. Opin. Biotechnol. 12, 180–187 (2001).
Houdebaine, L. M. Transgenic animal bioreactors. Transgenic Res. 9, 305–320 (2000).
Fischer, R. & Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 9, 279–299 (2000).
Giddings, G. Transgenic plants as protein factories. Curr. Opin. Biotechnol. 12, 450–454 (2001).
Barta, A. et al. The expression of a nopaline synthase human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 6, 347–357 (1986).
Hiatt, A., Cafferkey, R. & Bowdish, K. Production of antibodies in transgenic plants. Nature 342, 76–78 (1989).
Mason, H. S., Lam, D. M. K. & Arntzen, C. J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl Acad. Sci. USA 89, 11745–11749 (1992).
Thanavala, Y., Yang, Y. -F., Lyons, P., Mason, H. S. & Arntzen, C. J. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc. Natl Acad. Sci. USA 92, 3358–3361 (1995).
Hood, E. E. et al. Criteria for high-level expression of a fungal laccase gene in transgenic maize. Plant Biotechnol. J. 1, 129–140 (2003).
Hood, E. E. et al. Commercial production of avidin from transgenic maize: characterization of transformant, production, processing, extraction and purification. Mol. Breeding 3, 291–306 (1997).
Chong, D. K. X. et al. Expression of the human milk protein β-casein in transgenic potato plants. Transgenic Res. 6, 289–296 (1997).
Ruggiero, F. et al. Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 469, 132–136 (2000).
Staub, J. M. et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nature Biotechnol. 18, 333–338 (2000). This report shows that the secreted human protein somatotropin is soluble and biologically active when expressed in tobacco chloroplasts and has correctly formed disulphide bonds.
Fernandez-San Millan, A., Mingo-Castel, A., Miller, M. & Daniell, H. A chloroplast transgenic approach to hyper-express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. 1, 77–79 (2003).
Moloney, M., Boothe, J. & Van Rooijen, G. Oil bodies and associated proteins as affinity matrices. US Patent 6,509,453 (2003)
Chadd, H. E. & Chamow, S. M. Therapeutic antibody expression technology. Curr. Opin. Biotechnol. 12, 188–194 (2001).
Ma, J. K. et al. Generation and assembly of secretory antibodies in plants. Science 268, 716–719 (1995). This study shows for the first time that secretory antibodies, with 10 polypeptide chains that represent the products of four genes, can be assembled correctly in transgenic plants. Two rounds of crossing, which involved four singly-transgenic lines, were required to stack all four transgenes in the same plant.
Richter, L. J., Thanavala, Y., Arntzen, C. J. & Mason, H. S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nature Biotechnol. 18, 1167–1171 (2000).
Kapusta, J. et al. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 13, 1796–1799 (1999).
Tacket, C. O. et al. Immunogenicity in humans of a recombinant bacterial-antigen delivered in a transgenic potato. Nature Med. 4, 607–609 (1998).
Tacket, C. O. et al. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 182, 302–305 (2000).
Chong, D. K. X. & Langridge, W. H. R. Expression of full-length bioactive antimicrobial human lactoferrin in potato plants. Transgenic Res. 9, 71–78 (2000).
Zhang, X., Urry, D. W. & Daniell, H. Expression of an environmentally friendly synthetic protein-based polymer in transgenic tobacco plants. Plant Cell Reps. 16, 174–179 (1996).
Guda, C., Lee, S. B. & Daniell, H. Stable expression of a biodegradable protein-based polymer in stable tobacco chloroplasts. Plant Cell Reps. 19, 257–262 (2000).
Merle, C. et al. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett. 515, 114–118 (2002).
Scheller, J., Guhrs, K. H., Grosse, F. & Conrad, U. Production of spider silk proteins in tobacco and potato. Nature Biotechnol. 19, 573–577 (2001).
O'Dell, J. T., Nagy, F. & Chua, N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313, 810–812 (1985).
Lawton, M. A. et al. Expression of a soybean β-conclycinin gene under the control of the cauliflower mosaic virus 35S and 19S promoters in transformed petunia tissues. Plant Mol. Biol. 9, 315–324 (1987).
Kay, R., Chan, A., Daly, M. & McPherson, J. Duplication of CaMV-35S promoter sequences creates a strong enhancer for plant genes. Science 236, 1299–1302 (1987).
Christensen, A. H. & Quail, P. H. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 5, 213–218 (1996).
Vain, P., Finer, K. R., Engler, D. E., Pratt, R. C. & Finer, J. J. Intron-mediated enhancement of gene expression in maize (Zea mays L.) and bluegrass (Poa pratensis L.). Plant Cell Rep. 15, 489–494 (1996).
Stoger, E. et al. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Mol. Biol. 42, 583–590 (2000).
Artsaenko, O., Kettig, B., Fiedler, U., Conrad, U. & Düring K. Potato tubers as a biofactory for recombinant antibodies. Mol. Breeding 4, 313–319 (1998).
Padidam, M. Chemically regulated gene expression in plants. Curr. Opin. Plant Biol. 6, 169–177 (2003).
Padidam, M., Gore, M., Lu, D. L. & Smirnova, O. Chemical-inducible, ecdysone receptor-based gene expression system for plants. Transgenic Res. 12, 101–109 (2003).
Cramer, C. L., Boothe, J. G. & Oishi, K. K. Transgenic plants for therapeutic proteins: linking upstream and downstream technologies. Curr. Top. Microbiol. Immunol. 240, 95–118 (1999).
Schillberg, S., Zimmermann, S., Voss, A. & Fischer, R. Apoplastic and cytosolic expression of full-size antibodies and antibody fragments in Nicotiana tabacum. Transgenic Res. 8, 255–263 (1999). This paper compares the stability of identical scFv antibodies that are targeted to different compartments, and shows that the secretory pathway is generally much more suitable for antibody accumulation than the cytosol.
De Jaeger, G. et al. High-level accumulation of single-chain variable fragments in the cytosol of transgenic. Petunia hybrida. Eur. J. Biochem. 259, 1–10 (1998).
Schouten, A., Rossien, J., Bakker, J. & Schots, A. Formation of disulfide bridges by a single-chain Fv antibody in the reducing ectopic environment of the plant cytosol. J. Biol. Chem. 277, 19339–19345 (2002).
Conrad, U. & Fiedler, U. Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol. Biol. 38, 101–109 (1998).
Plasterk, R. H. A. & Ketting, R. F. The silence of the genes. Curr. Opin. Genet. Dev. 10, 562–567 (2000).
Anandalakshmi, R. et al. A viral suppressor of gene silencing in plants. Proc. Natl Acad. Sci. USA 95, 13079–13084 (1998).
Gelvin, S. B. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67, 16–23 (2003).
Britt, A. B. & May, G. D. Re-engineering plant gene targeting. Trends Plant Sci. 8, 90–95 (2003).
Veluthambi, K., Gupta, A. K. & Sharma, A. The current status of plant transformation technologies. Curr. Sci. India 84, 368–380 (2003).
Christou, P. Transformation technology. Trends Plant Sci. 1, 423–431 (1996).
Twyman, R. M., Stoger, E., Kohli, A. & Christou, P. in Genetic Engineering: Principles and Practice Vol. 24 (ed. Setlow, J. K.) 107–136 (Kluwer-Plenum, New York, 2002).
Martineau, B., Voelker, T. A. & Sanders, V. A. On defining T-DNA. Plant Cell 6, 1032–1033 (1994).
Hanson, B. et al. A simple method to enrich an Agrobacterium-transformed population for plants containing only T-DNA sequences. Plant J. 19, 727–734 (1999).
Fu, X. et al. Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res. 9, 11–19 (2000).
Lerouge, P., Bardor, M., Pagny, S., Gomord, V. & Faye, L. N-glycosylation of recombinant pharmaceutical glycoproteins produced in transgenic plants: towards humanisation of plant N-glycans. Curr. Pharmaceutical Biotech. 1, 347–354 (2000).
Bardor, M. et al. Immunoreactivity in mammals of two typical plant glyco-epitopes: core α(1,3)-fucose and core xylose. Glycobiology 13, 427–434 (2003).
Chargelegue, D., Vine, N. D., van Dolleweerd, C. J., Drake, P. M. & Ma, J. K. A murine monoclonal antibody produced in transgenic plants with plant-specific glycans is not immunogenic in mice. Transgenic Res. 9, 187–194 (2000). The first published paper that discusses the immunogenicity of a plant-derived glycosylated recombinant protein.
Warner, T. G. in Carbohydrates in Chemistry and Biology (eds Ernst, B., Hart, G. W. & Sanay, P.) 1043–1064 (Wiley, New York, 2000).
Blixt, O., Allin, K., Pereira, L., Datta, A. & Paulson, J. C. Efficient chemoenzymatic synthesis of O-linked sialyl oligosaccharides. J. Am. Chem. Soc. 124, 5739–5746 (2002).
Bakker, H. et al. Galactose-extended glycans of antibodies produced by transgenic plants. Proc. Natl Acad. Sci. USA 98, 2899–2904 (2001). In this study, a transgenic tobacco line that expressed the heavy and light chains of a murine antibody was crossed with a line that expressed human β-1,4-galactosyltransferase. The progeny produced antibodies ∼30% of which had partially galactosylated N-glycans, which provided a useful approach for the 'humanization' of plant glycans.
Raju, T. S., Briggs, J., Borge, S. M. & Jones, A. J. S. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10, 477–486 (2000). In this study, cell-specific glycosylation of immunoglobulins was studied by mass spectrometry and capillary electrophoresis/laser-induced fluorescence in 13 different animal systems. The glycan patterns were found to be unique in different species, which indicated that some might be more suitable than others for the production of human therapeutic proteins. The same might apply to plant systems, which were not considered in this paper.
Borisjuk, N. V. et al. Production of recombinant proteins in plant root exudates. Nature Biotechnol. 17, 466–469 (1999).
Komarnytsky, S., Borisjuk, N. V., Borisjuk, L. G., Alam, M. Z. & Raskin, I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 124, 927–933 (2000).
Drake, P. M. W. et al. Rhizosecretion of a monoclonal antibody protein complex from transgenic tobacco roots. Plant Mol. Biol. 52, 233–241 (2003).
Maliga, P. Engineering the plastid genome of higher plants. Curr. Opin. Plant Biol. 5, 164–172 (2002).
Daniell, H., Khan, M. S. & Allison, L. Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci. 7, 84–91 (2002).
Tregoning, J. S. et al. Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 31, 1174–1179 (2003).
Daniell, H., Lee, S. B., Panchal, T. & Wiebe, P. O. Expression of the native cholera B toxin subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 311, 1001–1009 (2001).
Khan, M. S. & Maliga, P. Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nature Biotechnol. 17, 910–915 (1999).
Fischer, R., Emans, N., Schuster, F., Hellwig, S. & Drossard, J. Towards molecular farming in the future: using plant-cell-suspension cultures as bioreactors. Biotechnol. Appl. Biochem. 30, 109–112 (1999).
Stöger, E. et al. Practical considerations for pharmaceutical antibody production in different crop systems. Mol. Breeding 9, 149–158 (2002). This paper considers in detail the factors that should be evaluated when choosing a crop system for the production of pharmaceutical proteins. The same scFv is expressed in many species to compare intrinsic yields, and features such as storage, distribution and biosafety are discussed, as well as economic factors.
Witcher, D. et al. Commercial production of β-glucuronidase (GUS): a model system for the production of proteins in plants. Mol. Breeding 4, 301–312 (1998).
Hood, E. E., Woodard, S. L. & Horn, M. E. Monoclonal antibody manufacturing in transgenic plants myths and realities. Curr. Opin. Biotechnol. 13, 630–635 (2002).
Hood, E. E. From green plants to industrial enzymes. Enzyme Microbial Technol. 30, 279–283 (2002).
Zeitlin, L. et al. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nature Biotechnol. 16, 1361–1364 (1998).
Khoudi, H. et al. Production of a diagnostic monoclonal antibody in perennial alfalfa plants. Biotechnol. Bioeng. 64, 135–143 (1999).
Perrin, Y. et al. Transgenic pea seeds as bioreactors for the production of a single-chain Fv fragment (scFV) antibody used in cancer diagnosis and therapy. Mol. Breeding 6, 345–352 (2000).
De Wilde, C., Peeters, K., Jacobs, A., Peck, I. & Depicker, A. Expression of antibodies and Fab fragments in transgenic potato plants: a case study for bulk production in crop plants. Mol. Breeding 9, 2871–282 (2002).
Schunmann, P. H. D., Coia, G. & Waterhouse, P. M. Biopharming the Simpli-RED™ HIV diagnostic reagent in barley, potato and tobacco. Mol. Breeding 9, 113–121 (2002).
McGarvey, P. B. et al. Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology 13, 1484–1487 (1995).
Sala, F. et al. Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives. Vaccine 21, 803–808 (2003).
Commandeur, U., Twyman, R. M. & Fischer, R. The biosafety of molecular farming in plants. AgBiotechNet 5, ABN 110 (2003).
Hare, P. D. & Chua, N. -H. Excision of selectable marker genes from transgenic plants. Nature Biotechnol. 20, 575–579 (2002).
Zuo, J. R., Niu, Q. W., Ikeda, Y. & Chua, N. H. Marker-free transformation: increasing transformation frequency by the use of regeneration-promoting genes. Curr. Opin. Biotechnol. 13, 173–180 (2002).
Eastham, K. & Sweet, J. Genetically Modified Organisms (GMOs): the Significance of Gene Flow through Pollen Transfer. Environment Issue Report No. 28 (European Environment Agency, Copenhagen, 2002)
Kay, E., Vogel, T. M., Bertolla, F., Nalin, R. & Simonet, P. In situ transfer of antibiotic resistance genes from transgenic (transplastomic) tobacco plants to bacteria. Appl. Environ. Microbiol. 68, 3345–3351 (2002).
Smalla, K. et al. in Proceedings of the 6th International Symposium on the Biosafety of Genetically Modified Organisms 146–154 (Univ. Extension Press, Univ. of Saskatchewan, Canada, 2000).
Smyth, S. & Phillips, P. W. B. Product differentiation alternatives: identity preservation, segregation and traceability. AgBioForum 5, 30–42 (2002).
Schillberg, S., Fischer, R. & Emans, N. Molecular farming of recombinant antibodies in plants. Cell. Mol. Life Sci. 60, 433–445 (2003). This is a comprehensive discussion of the technical issues concerning the production of antibodies in plants, which is treated in much more detail than is possible in the present review.
Stoger, E., Sack, M., Fischer, R. & Christou, P. Plantibodies: applications, advantages and bottlenecks. Curr. Opin. Biotechnol. 13, 161–166 (2002).
McCormick, A. A. et al. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants. Proc. Natl Acad. Sci. USA 96, 703–708 (1999).
Larrick, J. W., Yu, L., Naftzger, C., Jaiswal, S. & Wycoff, K. Production of secretory IgA antibodies in plants. Biomolecular Eng. 18, 87–94 (2001). This paper presents a useful summary of recent advances in the plant-based production of secretory IgAs with a discussion of purification methods and production costs.
Ma, J. K. et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nature Med. 4, 601–606 (1998).
Vaquero, C. et al. A carcinoembryonic antigen-specific diabody produced in tobacco. FASEB J. 16, 408–410 (2002).
Kathuria, S. et al. Efficacy of plant-produced recombinant antibodies against HCG. Human Reproduction 17, 2054–2061 (2002).
Miele, L. Plants as bioreactors for pharmaceuticals: regulatory considerations. Trends Biotechnol. 15, 45–50 (1997).
Emlay, D. in Plants as Factories for Protein Production (eds Hood, E. E. & Howard, J.) 175–180 (Kluwer Academic, New York, 2002).
Lloyd-Evans, M. & Nair, A. in Biopharming: the Emerging World Market of Plant-based Therapeutics. Theta Report No. 1214 63–80 (PJB Medical Publications Inc., New York, 2002).
Stoger, E., Schillberg, S., Twyman, R. M., Fischer, R. & Christou, P. in Methods in Molecular Biology. Antibody Engineering: Protocols and Methods 2nd edn (ed. Lo, B. K. C.) (Humana Press Inc., New Jersey, in the press).
Schillberg, S., Zimmermann, S., Findlay, K. & Fischer, R. Plasma membrane display of anti-viral single chain Fv fragments confers resistance to tobacco mosaic virus. Mol. Breeding 6, 317–326 (2000).
Sijmons, P. C. et al. Production of correctly processed human serum albumin in transgenic plants. Biotechnology 8, 217–221 (1990).
Zhu, Z. et al. Expression of human α-interferon in plants. Virology 172, 213–222 (1994).
Matsumoto, S., Ikura, K., Ueda, M. & Sasaki, R. Characterisation of a human glycoprotein (erythropoetin) produced in cultured tobacco cells. Plant Mol. Biol. 27, 1163–1172 (1995).
Delaney, D. et al. in Plant Biotechnology: 2002 and Beyond. Proceedings of the 10th IAPTC&B Congress, Orlando, Florida. (ed. Vasil, I.) 393–394 (Kluwer Academic, Dordrecht, The Netherlands, 2002).
Torres, E. et al. Rice cell culture as an alternative production system for functional diagnostic and therapeutic antibodies. Transgenic Res. 8, 441–449 (1999).
Terashima, M. et al. Production of functional human α1–antitrypsin by plant cell culture. Appl. Microbiol. Biotechnol. 52, 516–523 (1999).
Düring, K., Hippe, S., Kreuzaler, F. & Schell, J. Synthesis and self-assembly of a functional monoclonal antibody in transgenic Nicotiana tabacum. Plant Mol. Biol. 15, 281–293 (1990).
Ma, J. K.-C. et al. Generation and assembly of secretory antibodies in plants. Science 268, 716–719 (1995).
Francisco, J. A. et al. Expression and characterization of bryodin 1 and a bryodin 1-based single-chain immunotoxin from tobacco cell culture. Bioconjug. Chem. 8, 708–713 (1997).
Mayfield, S. P. et al. Expression and assembly of a fully active antibody in algae. Proc. Natl Acad. Sci. USA 100, 438–442 (2003).
Streatfield, S. J. et al. Plant-based vaccines: unique advantages. Vaccine 19, 2742–2748 (2001).
Ma, S. W. et al. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nature Med. 3, 793–796 (1997).
Yu, J. & Langridge, W. H. A plant-based multicomponent vaccine protects mice from enteric diseases. Nature Biotechnol. 19, 548–552 (2001).
Lamphear, B. J. et al. Delivery of subunit vaccines in maize seed. J. Control Release 83, 169–180 (2002).
Acknowledgements
The authors are grateful to R. Twyman for critical assessment and help with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- SINGLE-CHAIN FV FRAGMENTS
-
(scFvs). Monoclonal antibody derivatives that comprise a single polypeptide in which the variable regions of the heavy and light immunoglobulin chains are joined together by a flexible linker. scFvs are advantageous because only one transgene is required, and the molecules themselves are small and lack the effector functions of normal antibodies; however, a disadvantage is that they are univalent, whereas serum antibodies are divalent.
- MOLECULAR FARMING
-
The large-scale production of recombinant proteins in living cells or organisms; frequently applied to the use of crop plants or domestic animals as expression hosts because of the allusion to agriculture.
- HETEROLOGOUS
-
In the context of this article, a gene or protein that is not derived from the species in which it is expressed.
- TRANSPLASTOMIC
-
A transgenic plant in which the transgene is found in the plastid genome rather than the nuclear genome.
- DIABODY
-
A recombinant antibody that comprises the heavy- and light-chain variable regions joined by a flexible peptide linker. The linker is long enough to allow separation of the domains so that two of the polypeptides can assemble into a dimer, making the antibody divalent.
- MINIBODY
-
A recombinant antibody in which the heavy- and light-chain variable regions are part of the same polypeptide chain, which also includes the heavy-chain hinge region and one heavy-chain constant domain.
- AGROINFILTRATED LEAVES
-
Usually leaves of tobacco (although many other species can be used) that are transiently transformed with Agrobacterium tumefaciens, which results in the transient expression of recombinant proteins. This is a useful strategy for testing expression constructs and obtaining small amounts of protein for analysis before going to the expense of transgenics.
- ENTEROTOXIGENIC
-
Producing toxins in the gut that specifically affect the intestinal mucosa.
- DICOLYLEDONOUS PLANTS
-
(Dicots). Broad-leaf flowering plants the seeds of which contain two cotyledons (embryonic seed leaves that either remain in the seed when the plant germinates or emerge and become green). Examples include potato, tomato, tobacco and all peas and beans.
- MONOCOTYLEDONOUS PLANTS
-
Narrow-leaf plants the seeds of which contain one cotyledon. Examples include cereals, grasses, orchids and lilies.
- EPITOPE
-
A single antigenic determinant on a protein that is recognized by an antibody. A single protein can have many epitopes.
- LEYDIG CELLS
-
Interstitial cells in the testis that are responsible for the production of male sex hormones, such as testosterone, and are important in male sexual differentiation.
- SITE-DIRECTED MUTAGENESIS
-
An in vitro mutagenesis procedure that is often carried out using the polymerase chain reaction in which specific mutations are introduced into a DNA molecule.
- SIGNAL PEPTIDE
-
A short sequence of mainly hydrophobic amino acids at the N-terminus of secreted proteins. This peptide is captured by a signal-recognition particle as it emerges from the ribosome, which allows the ribosome to be transported to the endoplasmic reticulum.
- MOLECULAR CHAPERONES
-
Proteins the function of which is to ensure correct folding of other proteins during or after synthesis, or the refolding of denatured proteins.
- APOPLAST
-
The extracellular space. In plants, this is a large and continuous network of cavities under the cell wall. Proteins that are secreted from the cell often remain trapped here.
- POSITION EFFECTS
-
When transgenes integrate into genomic DNA, the expression level is often influenced by the surrounding chromatin. Local regulatory elements, such as enhancers, also influence transgene expression. Position effects lead to wide variations in transgene expression levels, even in plants that are transformed with identical constructs.
- AFFINITY TAGS
-
Short peptide sequences added to recombinant proteins, which bind strongly to particular affinity matrices and can be used to purify recombinant proteins.
- AGROBACTERIUM-MEDIATED TRANSFORMATION
-
Transformation that is achieved using the natural gene-transfer mechanism of Agrobacterium tumefaciens.
- WHISKER TRANSFORMATION
-
Transformation that is achieved by mixing walled plant cells with silicon carbide fibres that penetrate the cell wall and membrane, which generate pores through which DNA can be taken up into the cell.
- ELECTROPORATION
-
Transformation that is achieved by exposing cells or protoplasts to a brief pulse of electricity, which results in the formation of transient membrane pores through which DNA can be taken up into the cell.
- PROTOPLAST TRANSFORMATION
-
Any technique for introducing DNA into unwalled plant cells (protoplasts), such as calcium phosphate transfection, PEG transfection or electroporation.
- T-DNA BORDER SEQUENCES
-
Imperfect 25 bp direct repeat sequences that flank the piece of DNA that is transferred to the plant genome by Agrobacterium tumefaciens. These sequences are recognized by the bacterial VIRD1 and VIRD2 proteins, which form an endonuclease complex. Cleavage of the border sequences initiates T-DNA transfer.
- HYBRIDOMA CELLS
-
A hybrid cell line that is created by fusing a mortal antibody-producing B-lymphocyte with an immortalized myeloma line. The hybridoma line is immortal and produces a continuous supply of a particular monoclonal antibody.
- SOLANACEAE
-
A family of flowering plants (order Solanales) that comprise ∼100 genera and ∼2,500 species, many of which are economically important as food or medicinal crops. Examples include tobacco, potato and tomato.
- FRIABLE CALLUS TISSUE
-
Callus tissue is undifferentiated plant tissue, which grows when seeds or explants are cultured on media that contains an appropriate balance of plant hormones. Friable callus tissue is easily broken into fragments.
- BATCH, FED-BATCH, PERFUSION AND CONTINUOUS FERMENTATION
-
Batch fermentation is a closed system in which all of the substrate is added at the beginning, whereas in the fed-batch process the substrate is added in increments as fermentation proceeds. Continuous fermentation is an open system in which substrate is added continuously at a steady rate. Perfusion fermentation is a continuous process that allows cells to be grown at high density, and so results in increased biomass and product yields.
- RHIZOSPHERE
-
The soil zone that surrounds plant roots, which is rich in microorganisms and in which interactions occur between plants and microbes.
- LEAF GUTTATION FLUID
-
Fluid that seeps from the apoplast onto the leaf surface. In plants with large leaves, such as tobacco, large amounts of guttation fluid can be produced each day.
Rights and permissions
About this article
Cite this article
Ma, JC., Drake, P. & Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4, 794–805 (2003). https://doi.org/10.1038/nrg1177
Issue Date:
DOI: https://doi.org/10.1038/nrg1177
This article is cited by
-
High-yield BMP2 expression in rice cells via CRISPR and endogenous αAmy3 promoter
Applied Microbiology and Biotechnology (2024)
-
Biosynthesis of hyaluronic acid in Nicotiana tabacum plants: an efficient and safe method for large-scale production
Journal of Crop Science and Biotechnology (2024)
-
Efficient in planta production of amidated antimicrobial peptides that are active against drug-resistant ESKAPE pathogens
Nature Communications (2023)
-
Plant molecular farming in the wake of the closure of Medicago Inc
Nature Biotechnology (2023)
-
Recombinant anti-HIV MAP30, a ribosome inactivating protein: against plant virus and bacteriophage
Scientific Reports (2023)