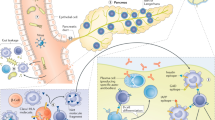
Key Points
-
Viral infections — particularly by enteroviruses (for example, coxsackievirus) — have been implicated in the development of type 1 diabetes mellitus (T1DM)
-
Many candidate genes for T1DM regulate antiviral responses in pancreatic β cells
-
Pancreatic islet α cells trigger a more efficient antiviral response than β cells following infection with diabetogenic viruses, thus enabling α cells to eradicate viral infections without undergoing apoptosis
-
An inability to clear viral infections could explain why chronically infected β cells, but not α cells, are targeted by an autoimmune response and killed during development of T1DM
-
The identification of key diabetogenic viruses and the downstream mechanisms leading to insulitis might enable a preventive approach to T1DM by vaccination
Abstract
Type 1 diabetes mellitus (T1DM) is caused by progressive autoimmune-mediated loss of pancreatic β-cell mass via apoptosis. The onset of T1DM depends on environmental factors that interact with predisposing genes to induce an autoimmune assault against β cells. Epidemiological, clinical and pathology studies in humans support viral infection — particularly by enteroviruses (for example, coxsackievirus) — as an environmental trigger for the development of T1DM. Many candidate genes for T1DM, such as MDA5, PTPN2 and TYK2, regulate antiviral responses in both β cells and the immune system. Cellular permissiveness to viral infection is modulated by innate antiviral responses that vary among different tissues or cell types. Some data indicate that pancreatic islet α cells trigger a more efficient antiviral response to infection with diabetogenic viruses than do β cells, and so are able to eradicate viral infections without undergoing apoptosis. This difference could account for the varying ability of islet-cell subtypes to clear viral infections and explain why chronically infected pancreatic β cells, but not α cells, are targeted by an autoimmune response and killed during the development of T1DM. These issues and attempts to target viral infection as a preventive therapy for T1DM are discussed in the present Review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Eizirik, D. L., Colli, M. L. & Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 5, 219–226 (2009).
Butler, A. E. et al. Modestly increased β-cell apoptosis but no increased β-cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 50, 2323–2331 (2007).
Meier, J. J., Bhushan, A., Butler, A. E., Rizza, R. A. & Butler, P. C. Sustained β-cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48, 2221–2228 (2005).
Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14, 619–633 (1965).
Kloppel, G., Drenck, C. R., Oberholzer, M. & Heitz, P. U. Morphometric evidence for a striking β-cell reduction at the clinical onset of type 1 diabetes. Virchows Arch. A Pathol. Anat. Histopathol. 403, 441–452 (1984).
Foulis, A. K. & Stewart, J. A. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26, 456–461 (1984).
Krogvold, L. et al. Function of isolated pancreatic islets fom patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD study. Diabetes 64, 2506–2512 (2015).
Strandell, E., Eizirik, D. L. & Sandler, S. Reversal of β-cell suppression in vitro in pancreatic islets isolated from nonobese diabetic mice during the phase preceding insulin-dependent diabetes mellitus. J. Clin. Invest. 85, 1944–1950 (1990).
Campbell-Thompson, M. et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 65, 719–731 (2016).
Todd, J. A. Etiology of type 1 diabetes. Immunity 32, 457–467 (2010).
Santin, I. & Eizirik, D. L. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes. Metab. 15 (Suppl. 3), 71–81 (2013).
Patterson, C. C., Dahlquist, G. G., Gyurus, E., Green, A. & Soltesz, G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 373, 2027–2033 (2009).
Bodansky, H. J., Staines, A., Stephenson, C., Haigh, D. & Cartwright, R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 304, 1020–1022 (1992).
Redondo, M. J., Jeffrey, J., Fain, P. R., Eisenbarth, G. S. & Orban, T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 359, 2849–2850 (2008).
Richardson, S. J., Morgan, N. G. & Foulis, A. K. Pancreatic pathology in type 1 diabetes mellitus. Endocr. Pathol. 25, 80–92 (2014).
Kondrashova, A. & Hyoty, H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int. Rev. Immunol. 33, 284–295 (2014).
Drescher, K. M., von Herrath, M. & Tracy, S. Enteroviruses, hygiene and type 1 diabetes: toward a preventive vaccine. Rev. Med. Virol. 25, 19–32 (2015).
Hober, D. & Sauter, P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat. Rev. Endocrinol. 6, 279–289 (2010).
Ghazarian, L., Diana, J., Simoni, Y., Beaudoin, L. & Lehuen, A. Prevention or acceleration of type 1 diabetes by viruses. Cell. Mol. Life Sci. 70, 239–255 (2013).
Imagawa, A. & Hanafusa, T. Fulminant type 1 diabetes — an important subtype in East Asia. Diabetes Metab. Res. Rev. 27, 959–964 (2011).
Tanaka, S., Aida, K., Nishida, Y. & Kobayashi, T. Pathophysiological mechanisms involving aggressive islet cell destruction in fulminant type 1 diabetes. Endocr. J. 60, 837–845 (2013).
Gamble, D. R. & Taylor, K. W. Seasonal incidence of diabetes mellitus. Br. Med. J. 3, 631–633 (1969).
Schulte, B. M. et al. Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol. 23, 99–104 (2010).
Gamble, D. R., Kinsley, M. L., FitzGerald, M. G., Bolton, R. & Taylor, K. W. Viral antibodies in diabetes mellitus. Br. Med. J. 3, 627–630 (1969).
Oikarinen, S. et al. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes 63, 655–662 (2014).
Laitinen, O. H. et al. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes 63, 446–455 (2014).
Yeung, W. C., Rawlinson, W. D. & Craig, M. E. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342, d35 (2011).
Ylipaasto, P. et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet β-cells. Diabetologia 47, 225–239 (2004).
Dotta, F. et al. Coxsackie B4 virus infection of β-cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl Acad. Sci. USA 104, 5115–5120 (2007).
Richardson, S. J., Leete, P., Bone, A. J., Foulis, A. K. & Morgan, N. G. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 56, 185–193 (2013).
Richardson, S. J., Willcox, A., Bone, A. J., Foulis, A. K. & Morgan, N. G. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52, 1143–1151 (2009).
Krogvold, L. et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 64, 1682–1687 (2015).
Chapman, N. M. & Kim, K. S. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr. Top. Microbiol. Immunol. 323, 275–292 (2008).
Tracy, S., Smithee, S., Alhazmi, A. & Chapman, N. Coxsackievirus can persist in murine pancreas by deletion of 5′ terminal genomic sequences. J. Med. Virol. 87, 240–247 (2015).
Willcox, A., Richardson, S. J., Bone, A. J., Foulis, A. K. & Morgan, N. G. Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia 54, 2417–2420 (2011).
Oikarinen, M. et al. Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 51, 1796–1802 (2008).
Cnop, M. et al. The long lifespan and low turnover of human islet β-cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53, 321–330 (2010).
Bonifacio, E., Lampasona, V., Genovese, S., Ferrari, M. & Bosi, E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J. Immunol. 155, 5419–5426 (1995).
Coppieters, K. T. et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012).
Savinov, A. Y., Wong, F. S., Stonebraker, A. C. & Chervonsky, A. V. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J. Exp. Med. 197, 643–656 (2003).
Chabot, S. et al. Mouse liver-specific CD8+ T-cells encounter their cognate antigen and acquire capacity to destroy target hepatocytes. J. Autoimmun. 42, 19–28 (2013).
Christen, U. et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J. Clin. Invest. 113, 74–84 (2004).
Filippi, C. M., Estes, E. A., Oldham, J. E. & von Herrath, M. G. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 119, 1515–1523 (2009).
Drescher, K. M., Kono, K., Bopegamage, S., Carson, S. D. & Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329, 381–394 (2004).
Flodstrom, M. et al. Target cell defense prevents the development of diabetes after viral infection. Nat. Immunol. 3, 373–382 (2002).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).
Horwitz, M. S. et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4, 781–785 (1998).
Sarmiento, L., Cubas-Duenas, I. & Cabrera-Rode, E. Evidence of association between type 1 diabetes and exposure to enterovirus in Cuban children and adolescents. MEDICC Rev. 15, 29–32 (2013).
Chehadeh, W. et al. Persistent infection of human pancreatic islets by coxsackievirus B is associated with α-interferon synthesis in β-cells. J. Virol. 74, 10153–10164 (2000).
Marroqui, L. et al. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic β-cells. Diabetes 64, 3808–3811 (2015).
Slifka, M. K., Rodriguez, F. & Whitton, J. L. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401, 76–79 (1999).
Campbell, I. L., Kay, T. W., Oxbrow, L. & Harrison, L. C. Essential role for interferon-γ and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J. Clin. Invest. 87, 739–742 (1991).
Kay, T. W., Campbell, I. L., Oxbrow, L. & Harrison, L. C. Overexpression of class I major histocompatibility complex accompanies insulitis in the non-obese diabetic mouse and is prevented by anti-interferon-γ antibody. Diabetologia 34, 779–785 (1991).
von Herrath, M. G. & Oldstone, M. B. Interferon-γ is essential for destruction of β-cells and development of insulin-dependent diabetes mellitus. J. Exp. Med. 185, 531–539 (1997).
Eizirik, D. L. & Mandrup-Poulsen, T. A choice of death — the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 44, 2115–2133 (2001).
Sandberg, J. O., Eizirik, D. L. & Sandler, S. IL-1 receptor antagonist inhibits recurrence of disease after syngeneic pancreatic islet transplantation to spontaneously diabetic non-obese diabetic (NOD) mice. Clin. Exp. Immunol. 108, 314–317 (1997).
Gurzov, E. N. & Eizirik, D. L. Bcl-2 proteins in diabetes: mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol. 21, 424–431 (2011).
Atkinson, M. A. et al. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J. Clin. Invest. 94, 2125–2129 (1994).
Honeyman, M. C., Stone, N. L. & Harrison, L. C. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol. Med. 4, 231–239 (1998).
Hiemstra, H. S. et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA 98, 3988–3991 (2001).
Richter, W. et al. Sequence homology of the diabetes-associated autoantigen glutamate decarboxylase with coxsackie B4-2C protein and heat shock protein 60 mediates no molecular mimicry of autoantibodies. J. Exp. Med. 180, 721–726 (1994).
Schloot, N. C. et al. Molecular mimicry in type 1 diabetes mellitus revisited: T-cell clones to GAD65 peptides with sequence homology to coxsackie or proinsulin peptides do not crossreact with homologous counterpart. Hum. Immunol. 62, 299–309 (2001).
Christen, U. et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Invest. 114, 1290–1298 (2004).
Ehl, S., Hombach, J., Aichele, P., Hengartner, H. & Zinkernagel, R. M. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J. Exp. Med. 185, 1241–1251 (1997).
Zarozinski, C. C. & Welsh, R. M. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J. Exp. Med. 185, 1629–1639 (1997).
Pane, J. A. & Coulson, B. S. Lessons from the mouse: potential contribution of bystander lymphocyte activation by viruses to human type 1 diabetes. Diabetologia 58, 1149–1159 (2015).
Marroqui, L. et al. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic α- and β-cells. eLIFE 4, e06990 (2015).
Serreze, D. V. et al. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and γ interferon. J. Virol. 79, 1045–1052 (2005).
Larsson, P. G. et al. A preclinical study on the efficacy and safety of a new vaccine against Coxsackievirus B1 reveals no risk for accelerated diabetes development in mouse models. Diabetologia 58, 346–354 (2015).
Serreze, D. V., Ottendorfer, E. W., Ellis, T. M., Gauntt, C. J. & Atkinson, M. A. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 49, 708–711 (2000).
Fattovich, G., Giustina, G., Favarato, S. & Ruol, A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with α interferon. J. Hepatol. 24, 38–47 (1996).
Nakamura, K. et al. Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 34, 2084–2089 (2011).
Zornitzki, T., Malnick, S., Lysyy, L. & Knobler, H. Interferon therapy in hepatitis C leading to chronic type 1 diabetes. World J. Gastroenterol. 21, 233–239 (2015).
Casadevall, A. & Pirofski, L. A. Microbiology: ditch the term pathogen. Nature 516, 165–166 (2014).
Pociot, F. et al. Genetics of type 1 diabetes: what's next? Diabetes 59, 1561–1571 (2010).
Bonifacio, E., Krumsiek, J., Winkler, C., Theis, F. J. & Ziegler, A. G. A strategy to find gene combinations that identify children who progress rapidly to type 1 diabetes after islet autoantibody seroconversion. Acta Diabetol. 51, 403–411 (2014).
Chen, Y. G. et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes 63, 3960–3973 (2014).
Onengut-Gumuscu, S. et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat. Genet. 47, 381–386 (2015).
Concannon, P., Rich, S. S. & Nepom, G. T. Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654 (2009).
Eizirik, D. L. et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 8, e1002552 (2012).
Bergholdt, R. et al. Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes 61, 954–962 (2012).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Moore, F. et al. PTPN2, a candidate gene for type 1 diabetes, modulates interferon-γ-induced pancreatic β-cell apoptosis. Diabetes 58, 1283–1291 (2009).
Colli, M. L., Moore, F., Gurzov, E. N., Ortis, F. & Eizirik, D. L. MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic β-cell responses to the viral by-product double-stranded RNA. Hum. Mol. Genet. 19, 135–146 (2010).
Santin, I. et al. PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic β-cell apoptosis via regulation of the BH3-only protein Bim. Diabetes 60, 3279–3288 (2011).
Nogueira, T. C. et al. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic β-cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 9, e1003532 (2013).
Floyel, T. et al. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl Acad. Sci. USA 111, 10305–10310 (2014).
Marroqui, L. et al. BACH2, a candidate risk gene for type 1 diabetes, regulates apoptosis in pancreatic β-cells via JNK1 modulation and crosstalk with the candidate gene PTPN2. Diabetes 63, 2516–2527 (2014).
Coccia, E. M. & Battistini, A. Early IFN type I response: learning from microbial evasion strategies. Semin. Immunol. 27, 85–101 (2015).
Huang, X. et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes 44, 658–664 (1995).
Ferreira, R. C. et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 63, 2538–2550 (2014).
Reynier, F. et al. Specific gene expression signature associated with development of autoimmune type-I diabetes using whole-blood microarray analysis. Genes Immun. 11, 269–278 (2010).
Li, Q. et al. Interferon-α initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 12439–12444 (2008).
Diana, J. et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 19, 65–73 (2013).
Quah, H. S. et al. Deficiency in type I interferon signaling prevents the early interferon-induced gene signature in pancreatic islets but not type 1 diabetes in NOD mice. Diabetes 63, 1032–1040 (2014).
Nejentsev, S., Walker, N., Riches, D., Egholm, M. & Todd, J. A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 (2009).
Downes, K. et al. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS ONE 5, e12646 (2010).
Winkler, C. et al. An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes 60, 685–690 (2011).
Lempainen, J. et al. Non-HLA gene effects on the disease process of type 1 diabetes: from HLA susceptibility to overt disease. J. Autoimmun. 61, 45–53 (2015).
Lincez, P. J., Shanina, I. & Horwitz, M. S. Reduced expression of the MDA5 gene IFIH1 prevents autoimmune diabetes. Diabetes 64, 2184–2193 (2015).
Wallace, C. et al. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat. Genet. 42, 68–71 (2010).
Tao, J. H. et al. Meta-analysis of TYK2 gene polymorphisms association with susceptibility to autoimmune and inflammatory diseases. Mol. Biol. Rep. 38, 4663–4672 (2011).
Richter, M. F., Dumenil, G., Uze, G., Fellous, M. & Pellegrini, S. Specific contribution of Tyk2 JH regions to the binding and the expression of the interferon α/β receptor component IFNAR1. J. Biol. Chem. 273, 24723–24729 (1998).
Izumi, K. et al. Reduced Tyk2 gene expression in β-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat. Commun. 6, 6748 (2015).
Nagafuchi, S. et al. TYK2 promoter variant and diabetes mellitus in the Japanese. EBioMedicine 2, 744–749 (2015).
Smyth, D. J. et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N. Engl. J. Med. 359, 2767–2777 (2008).
Espino-Paisan, L. et al. A polymorphism in PTPN2 gene is associated with an earlier onset of type 1 diabetes. Immunogenetics 63, 255–258 (2011).
Long, S. A. et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun. 12, 116–125 (2011).
Zhernakova, A. et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 7, e1002004 (2011).
Heinig, M. et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 467, 460–464 (2010).
Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008).
Santin, I. et al. USP18 is a key regulator of the interferon-driven gene network modulating pancreatic β-cell inflammation and apoptosis. Cell Death Dis. 3, e419 (2012).
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989).
Kamradt, T., Goggel, R. & Erb, K. J. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 26, 260–267 (2005).
Kondrashova, A., Seiskari, T., Ilonen, J., Knip, M. & Hyoty, H. The 'hygiene hypothesis' and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. Apmis 121, 478–493 (2013).
Cooke, A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of type 1 diabetes? Immunology 126, 12–17 (2009).
Bach, J. F. & Chatenoud, L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb. Perspect. Med. 2, a007799 (2012).
Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501–513 (2010).
Rook, G. A. & Brunet, L. R. Old friends for breakfast. Clin. Exp. Allergy 35, 841–842 (2005).
Gulden, E., Wong, F. S. & Wen, L. The gut microbiota and type 1 diabetes. Clin. Immunol. 159, 143–153 (2015).
Ziegler, A. G. et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309, 2473–2479 (2013).
Krischer, J. P. et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 58, 980–987 (2015).
Ilonen, J. et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 62, 3636–3640 (2013).
Vercelli, D. Mechanisms of the hygiene hypothesis — molecular and otherwise. Curr. Opin. Immunol. 18, 733–737 (2006).
Morgan, N. G. & Richardson, S. J. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol. Metab. 25, 611–619 (2014).
Randow, F., MacMicking, J. D. & James, L. C. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340, 701–706 (2013).
Yan, N. & Chen, Z. J. Intrinsic antiviral immunity. Nat. Immunol. 13, 214–222 (2012).
Cnop, M. et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes 63, 1978–1993 (2014).
Carty, M., Reinert, L., Paludan, S. R. & Bowie, A. G. Innate antiviral signalling in the central nervous system. Trends Immunol. 35, 79–87 (2014).
Marroqui, L. et al. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. EBioMedicine 2, 378–385 (2015).
Anagandula, M. et al. Infection of human islets of Langerhans with two strains of coxsackie B virus serotype 1: assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J. Med. Virol. 86, 1402–1411 (2014).
Gallagher, G. R. et al. Viral infection of engrafted human islets leads to diabetes. Diabetes 64, 1358–1369 (2015).
Cho, H. et al. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat. Med. 19, 458–464 (2013).
Atkinson, M. A., von Herrath, M., Powers, A. C. & Clare-Salzler, M. Current concepts on the pathogenesis of type 1 diabetes — considerations for attempts to prevent and reverse the disease. Diabetes Care 38, 979–988 (2015).
Hyoty, H. & Knip, M. Developing a vaccine for type 1 diabetes through targeting enteroviral infections. Expert Rev. Vaccines 13, 989–999 (2014).
De Palma, A. M., Vliegen, I., De Clercq, E. & Neyts, J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 28, 823–884 (2008).
Moell, A., Skog, O., Ahlin, E., Korsgren, O. & Frisk, G. Antiviral effect of nicotinamide on enterovirus-infected human islets in vitro: effect on virus replication and chemokine secretion. J. Med. Virol. 81, 1082–1087 (2009).
Berg, A. K., Olsson, A., Korsgren, O. & Frisk, G. Antiviral treatment of coxsackie B virus infection in human pancreatic islets. Antiviral Res. 74, 65–71 (2007).
See, D. M. & Tilles, J. G. WIN 54954 treatment of mice infected with a diabetogenic strain of group B coxsackievirus. Antimicrob. Agents Chemother. 37, 1593–1598 (1993).
Powers, R. D., Dotson, W. M. Jr & Hayden, F. G. Modification of encephalomyocarditis virus-induced diabetes in mice by antiviral agents. Antiviral Res. 3, 151–159 (1983).
Alidjinou, E. K., Sane, F., Bertin, A., Caloone, D. & Hober, D. Persistent infection of human pancreatic cells with coxsackievirus B4 is cured by fluoxetine. Antiviral Res. 116, 51–54 (2015).
Schneider, D. A. & von Herrath, M. G. Potential viral pathogenic mechanism in human type 1 diabetes. Diabetologia 57, 2009–2018 (2014).
Sin, J., Mangale, V., Thienphrapa, W., Gottlieb, R. A. & Feuer, R. Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis. Virology 484, 288–304 (2015).
Acknowledgements
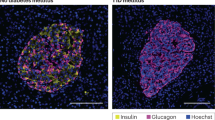
The research by A.O.d.B. and D.L.E. that is discussed in this Review was supported by the Belgian Fonds National de la Recherche Scientifique (FNRS; grants T.0036.13 and FRFS-Welbio CR-2015A-06); the European Union (projects Naimit and BetaBat in the Seventh Framework Programme of the European Commission); the Juvenile Diabetes Foundation; the Helmsley Type 1 Diabetes Program; and the NIH–NIDDK–HIRN Consortium. A.O.d.B. and D.L.E. also receive support from the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). Organ procurement organisations partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/our-partners.php. F. Grieco (Center for Diabetes Research, Universite Libre de Bruxelles, Belgium) provided the micrographs shown in Fig. 3.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Op de Beeck, A., Eizirik, D. Viral infections in type 1 diabetes mellitus — why the β cells?. Nat Rev Endocrinol 12, 263–273 (2016). https://doi.org/10.1038/nrendo.2016.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.30
This article is cited by
-
Deficiency of Trex1 leads to spontaneous development of type 1 diabetes
Nutrition & Metabolism (2024)
-
The role of regulated necrosis in diabetes and its complications
Journal of Molecular Medicine (2024)
-
Interferons are key cytokines acting on pancreatic islets in type 1 diabetes
Diabetologia (2024)
-
Pre-treatment with IL-6 potentiates β-cell death induced by pro-inflammatory cytokines
BMC Molecular and Cell Biology (2023)
-
Identification of key regulatory genes and their working mechanisms in type 1 diabetes
BMC Medical Genomics (2023)