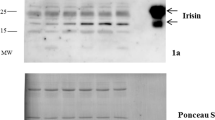
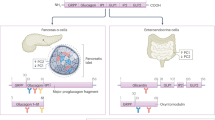
Key Points
-
Irisin is secreted primarily by muscle and in small amounts by adipose tissue
-
Irisin improves glucose homeostasis, lipid profile and metabolic parameters in animals and acts on adipose tissue by inducing 'browning', as well as on muscle and liver
-
Inconsistencies in published data regarding the circulating levels of irisin highlight the need for accurate methods for irisin measurement and for improved study design
-
Levels of irisin are increased in states of obesity and decreased in patients with type 2 diabetes mellitus (T2DM)
-
Future studies should try to identify the irisin receptor and to investigate the effects of irisin on the endocrine pancreas and appetite centres in the brain; prospective clinical studies should investigate whether irisin is a predictive marker for insulin resistance, T2DM or the metabolic syndrome
Abstract
Irisin is a myokine that leads to increased energy expenditure by stimulating the 'browning' of white adipose tissue. In the first description of this hormone, increased levels of circulating irisin, which is cleaved from its precursor fibronectin type III domain-containing protein 5, were associated with improved glucose homeostasis by reducing insulin resistance. Consequently, several studies attempted to characterize the role of irisin in glucose regulation, but contradictory results have been reported, and even the existence of this hormone has been questioned. In this Review, we present the current knowledge on the physiology of irisin and its role in glucose homeostasis. We describe the mechanisms involved in the synthesis, secretion, circulation and regulation of irisin, and the controversies regarding the measurement of irisin. We also discuss the direct effects of irisin on glucose regulatory mechanisms in different organs, the indirect effects and interactions with other hormones, and the important open questions with regard to irisin in those organs. Finally, we present the results from animal interventional studies and from human clinical studies investigating the association of irisin with obesity, insulin resistance, type 2 diabetes mellitus and the metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gerich, J. E. Physiology of glucose homeostasis. Diabetes Obes. Metab. 2, 345–350 (2000).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Bostrom, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012). This is the first study to describe the existence of irisin and its role in thermogenesis in mice.
NCBI. Fibronectin type III domain-containing protein 5 precursor [Rattus norvegicus]. NCBI https://www.ncbi.nlm.nih.gov/protein/NP_001257910.1 (2016).
NCBI. Fibronectin type III domain-containing protein 5 preproprotein [Mus musculus]. NCBI https://www.ncbi.nlm.nih.gov/protein/NP_081678.1 (2016).
NCBI. Fibronectin type III domain-containing protein 5 isoform 2 preproprotein [Homo sapiens]. NCBI https://www.ncbi.nlm.nih.gov/protein/NP_715637.2 (2016).
Schumacher, M. A., Chinnam, N., Ohashi, T., Shah, R. S. & Erickson, H. P. The structure of irisin reveals a novel intersubunit ß-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J. Biol. Chem. 288, 33738–33744 (2013).
Huh, J. Y. et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61, 1725–1738 (2012).
Roca-Rivada, A. et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 8, e60563 (2013).
Kurdiova, T. et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J. Physiol. 592, 1091–1107 (2014).
Moreno-Navarrete, J. M. et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 98, E769–E778 (2013).
Aydin, S. et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 61, 130–136 (2014).
Lv, J. et al. Study on the distribution and elimination of the new hormone irisin in vivo: new discoveries regarding irisin. Horm. Metab. Res. 47, 591–595 (2015).
Crujeiras, A. B., Pardo, M. & Casanueva, F. F. Irisin: 'fat' or artefact. Clin. Endocrinol. (Oxf.) 82, 467–474 (2015).
Elbelt, U., Hofmann, T. & Stengel, A. Irisin: what promise does it hold? Curr. Opin. Clin. Nutr. Metab. Care 16, 541–547 (2013).
Hofmann, T., Elbelt, U. & Stengel, A. Irisin as a muscle-derived hormone stimulating thermogenesis — a critical update. Peptides 54, 89–100 (2014).
Polyzos, S. A. & Mantzoros, C. S. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism 64, 937–942 (2015).
Polyzos, S. A., Mathew, H. & Mantzoros, C. S. Irisin: A true, circulating hormone. Metabolism 64, 1611–1618 (2015).
Sanchis-Gomar, F., Alis, R., Pareja-Galeano, H., Romagnoli, M. & Perez-Quilis, C. Inconsistency in circulating irisin levels: what is really happening? Horm. Metab. Res. 46, 591–596 (2014).
Raschke, S. et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 8, e73680 (2013). This was the first study questioning the existence and importance of irisin in humans.
Bostrom, P. A., Fernandez-Real, J. M. & Mantzoros, C. Irisin in humans: recent advances and questions for future research. Metabolism 63, 178–180 (2014).
Huh, J. Y., Siopi, A., Mougios, V., Park, K. H. & Mantzoros, C. S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 100, E453–E457 (2015).
Jedrychowski, M. P. et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 22, 734–740 (2015). This study is the latest confirmation from the investigators that discovered irisin showing that irisin exists in circulating levels in humans.
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014). This study further confirmed the existence of irisin in humans and its effects in browning.
Loffler, D. et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 100, 1289–1299 (2015).
Palacios-Gonzalez, B. et al. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obesity (Silver Spring) 23, 729–732 (2015).
Polyzos, S. A., Kountouras, J., Anastasilakis, A. D., Geladari, E. V. & Mantzoros, C. S. Irisin in patients with nonalcoholic fatty liver disease. Metabolism 63, 207–217 (2014).
Albrecht, E. et al. Irisin — a myth rather than an exercise-inducible myokine. Sci. Rep. 5, 8889 (2015). This is the second study, after the study conducted by Raschke, S. et al . (reference 21), that questioned the existence of irisin.
Kozak, M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol. Cell. Biol. 9, 5073–5080 (1989).
Bidlingmaier, M. & Freda, P. U. Measurement of human growth hormone by immunoassays: current status, unsolved problems and clinical consequences. Growth Horm. IGF Res. 20, 19–25 (2010).
Robinson, C. J., Gaines Das, R. & Woollacott, D. The first international standard for human leptin and the first international standard for mouse leptin: comparison of candidate preparations by in vitro bioassays and immunoassays. J. Mol. Endocrinol. 27, 69–76 (2001).
Sturgeon, C. M. & Ellis, A. R. Standardization of FSH, LH and hCG — current position and future prospects. Mol. Cell Endocrinol. 260–262, 301–309 (2007).
Sanchis-Gomar, F., Alis, R. & Lippi, G. Circulating irisin detection: does it really work? Trends Endocrinol. Metab. 26, 335–336 (2015).
Pilegaard, H., Saltin, B. & Neufer, P. D. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J. Physiol. 546, 851–858 (2003).
Spiegelman, B. M., Puigserver, P. & Wu, Z. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int. J. Obes. Relat. Metab. Disord. 24 (Suppl. 4), 8–10 (2000).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Kurdiova, T. et al. Exercise-mimicking treatment fails to increase Fndc5 mRNA and irisin secretion in primary human myotubes. Peptides 56, 1–7 (2014).
Peterson, J. M., Mart, R. & Bond, C. E. Effect of obesity and exercise on the expression of the novel myokines, Myonectin and Fibronectin type III domain containing 5. PeerJ 2, e605 (2014).
Norheim, F. et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 281, 739–749 (2014).
Besse-Patin, A. et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int. J. Obes. (Lond.) 38, 707–713 (2014).
Pekkala, S. et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 591, 5393–5400 (2013).
Al-Daghri, N. M. et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur. J. Clin. Invest. 45, 775–781 (2015).
Ellefsen, S. et al. Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur. J. Appl. Physiol. 114, 1875–1888 (2014).
Huh, J. Y. et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 99, E2154–E2161 (2014).
Huh, J. Y., Mougios, V., Skraparlis, A., Kabasakalis, A. & Mantzoros, C. S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 63, 918–921 (2014).
Ijiri, N., Kanazawa, H., Asai, K., Watanabe, T. & Hirata, K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology 20, 612–617 (2015).
Moraes, C. et al. Resistance exercise training does not affect plasma irisin levels of hemodialysis patients. Horm. Metab. Res. 45, 900–904 (2013).
Nygaard, H. et al. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE 10, e0121367 (2015).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Brenmoehl, J. et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 10, 338–349 (2014).
Tsuchiya, Y., Ando, D., Takamatsu, K. & Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 64, 1042–1050 (2015).
Huh, J. Y., Dincer, F., Mesfum, E. & Mantzoros, C. S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. (Lond.) 38, 1538–1544 (2014). This is the first study to describe the expression profile of FNDC5 (the precursor of irisin) and the factors predicting the circulating levels of irisin in humans.
Tiano, J. P., Springer, D. A. & Rane, S. G. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J. Biol. Chem. 290, 11431 (2015).
Tiano, J. P., Springer, D. A. & Rane, S. G. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J. Biol. Chem. 290, 7671–7684 (2015).
Yadav, H. et al. Protection from obesity and diabetes by blockade of TGF-ß/Smad3 signaling. Cell Metab. 14, 67–79 (2011).
Dong, J., Dong, Y., Chen, F., Mitch, W. E. & Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. (Lond.) 40, 434–442 (2016).
Shan, T., Liang, X., Bi, P. & Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 27, 1981–1989 (2013).
Rodriguez, A. et al. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int. J. Obes. (Lond.) 39, 397–407 (2015).
Li, M. et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 100, 1485–1493 (2015).
Gutierrez-Repiso, C. et al. FNDC5 could be regulated by leptin in adipose tissue. Eur. J. Clin. Invest. 44, 918–925 (2014).
Gavrieli, A., Panagiotou, G. & Mantzoros, C. S. Leptin administration in physiological or pharmacological doses does not alter circulating irisin levels in humans. Int. J. Obes. (Lond.) 40, 1461–1463 (2016).
Huerta, A. E. et al. Circulating irisin and glucose metabolism in overweight/obese women: effects of α-lipoic acid and eicosapentaenoic acid. J. Physiol. Biochem. 71, 547–558 (2015).
Koh, E. H. et al. Effects of α-lipoic acid on body weight in obese subjects. Am J. Med. 124, 85.e1–85.e8 (2011).
Rochette, L., Ghibu, S., Muresan, A. & Vergely, C. α-Lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 93, 1021–1027 (2015).
Matsuo, Y. et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J. Cachexia Sarcopenia Muscle 6, 62–72 (2015).
Rachid, T. L. et al. Fenofibrate (PPARα agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 402, 86–94 (2015).
Li, D. J. et al. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol. (Oxf.) 213, 711–721 (2015).
Yang, Z., Chen, X., Chen, Y. & Zhao, Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am. J. Transl Res. 7, 1850–1859 (2015).
Zhang, Y. et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63, 514–525 (2014). This study confirmed the initial findings of Bostrom, P. et al . (reference 4) and also provided a functional mechanism for the observed browning effects of irisin on WAT.
Elsen, M., Raschke, S. & Eckel, J. Browning of white fat: does irisin play a role in humans? J. Endocrinol. 222, R25–R38 (2014).
Zhang, Y. et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 311, E530–E541 (2016).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Xiong, X. Q. et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta 1852, 1867–1875 (2015).
Gao, S. et al. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS ONE 11, e0147480 (2016).
Lee, H. J. et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol. Endocrinol. 29, 873–881 (2015).
Xin, C. et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. (Lond.) 40, 443–451 (2016).
Yang, Z., Chen, X., Chen, Y. & Zhao, Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 8, 6490–6497 (2015).
Vaughan, R. A. et al. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 16, 711–718 (2014).
Vaughan, R. A., Gannon, N. P., Mermier, C. M. & Conn, C. A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 71, 679–689 (2015).
Liu, T. Y. et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. (Lond.) 129, 839–850 (2015).
Tang, H. et al. Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine 6, 139–148 (2016).
Mo, L. et al. Irisin is regulated by CAR in liver and is a mediator of hepatic glucose and lipid metabolism. Mol. Endocrinol. 30, 533–542 (2016).
Park, M. J., Kim, D. I., Choi, J. H., Heo, Y. R. & Park, S. H. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell. Signal. 27, 1831–1839 (2015).
Batirel, S., Bozaykut, P., Mutlu Altundag, E., Kartal Ozer, N. & Mantzoros, C. S. The effect of irisin on antioxidant system in liver. Free Radic. Biol. Med. 75 (Suppl. 1), S16 (2014).
Zhang, H. J. et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J. Hepatol. 59, 557–562 (2013).
Choi, E. S. et al. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PLoS ONE 9, e110680 (2014).
Rizk, F. H., Elshweikh, S. A. & Abd El-Naby, A. Y. Irisin levels in relation to metabolic and liver functions in Egyptian patients with metabolic syndrome. Can. J. Physiol. Pharmacol. 94, 359–362 (2016).
Polyzos, S. A., Kountouras, J., Anastasilakis, A. D., Margouta, A. & Mantzoros, C. S. Association between circulating irisin and homocysteine in patients with nonalcoholic fatty liver disease. Endocrine 49, 560–562 (2015).
Yang, M. et al. Circulating levels of irisin in middle-aged first-degree relatives of type 2 diabetes mellitus — correlation with pancreatic ß-cell function. Diabetol. Metab. Syndr. 6, 133 (2014).
Wang, L. et al. Circulating levels of betatrophin and irisin are not associated with pancreatic ß-cell function in previously diagnosed type 2 diabetes mellitus patients. J. Diabetes Res. 2016, 2616539 (2016).
Wen, M. S., Wang, C. Y., Lin, S. L. & Hung, K. C. Decrease in irisin in patients with chronic kidney disease. PLoS ONE 8, e64025 (2013).
Ebert, T. et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur. J. Endocrinol. 170, 501–506 (2014).
Liu, J. J. et al. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J. Diabetes Complicat. 28, 208–213 (2014).
Hu, W., Wang, R., Li, J., Zhang, J. & Wang, W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann. Clin. Biochem. 53, 67–74 (2016).
Wang, H. H., Zhang, X. W., Chen, W. K., Huang, Q. X. & Chen, Q. Q. Relationship between serum irisin levels and urinary albumin excretion in patients with type 2 diabetes. J. Diabetes Complicat. 29, 384–389 (2015).
Yang, S. et al. Association of serum irisin and body composition with chronic kidney disease in obese Chinese adults: a cross-sectional study. BMC Nephrol. 16, 16 (2015).
Dun, S. L. et al. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 240, 155–162 (2013).
Piya, M. K. et al. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 306, E512–E518 (2014).
Hashemi, M. S. et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231, 296–304 (2013).
Ghahrizjani, F. A. et al. Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene 557, 123–129 (2015).
Moon, H. S., Dincer, F. & Mantzoros, C. S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 62, 1131–1136 (2013).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013). This study links irisin and FNDC5 with neurocognitive function through a PGC1α–FNDC5–BDNF axis.
Zhang, W. et al. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc. Drugs Ther. 29, 121–127 (2015).
Zhang, W. et al. Irisin: A myokine with locomotor activity. Neurosci. Lett. 595, 7–11 (2015).
Schlogl, M. et al. Increased 24-hour ad libitum food intake is associated with lower plasma irisin concentrations the following morning in adult humans. Appetite 90, 154–159 (2015).
Han, F., Zhang, S., Hou, N., Wang, D. & Sun, X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am. J. Physiol. Heart Circ. Physiol. 309, H1501–H1508 (2015).
Lu, J. et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-null diabetic mice. Atherosclerosis 243, 438–448 (2015).
Zhu, G. et al. Irisin increased the number and improved the function of endothelial progenitor cells in diabetes mellitus mice. J. Cardiovasc. Pharmacol. 68, 67–73 (2016).
Song, H. et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS ONE 9, e110273 (2014).
Wu, F. et al. Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS ONE 10, e0134662 (2015).
Zhu, D. et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J. Mol. Cell. Cardiol. 87, 138–147 (2015).
Yi, P., Park, J. S. & Melton, D. A. Betatrophin: a hormone that controls pancreatic ß cell proliferation. Cell 153, 747–758 (2013).
Sanchis-Gomar, F. & Perez-Quilis, C. The p38–PGC-1α–irisin–betatrophin axis: exploring new pathways in insulin resistance. Adipocyte 3, 67–68 (2014).
Jiao, Y., Le Lay, J., Yu, M., Naji, A. & Kaestner, K. H. Elevated mouse hepatic betatrophin expression does not increase human ß-cell replication in the transplant setting. Diabetes 63, 1283–1288 (2014).
Cox, A. R. et al. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase ß cell proliferation in mice. Diabetologia 58, 1523–1531 (2015).
Gusarova, V. et al. ANGPTL8/betatrophin does not control pancreatic ß cell expansion. Cell 159, 691–696 (2014).
Yi, P., Park, J. -S. & Melton, D. A. Retraction notice to: betatrophin: a hormone that controls pancreatic ß cell proliferation. Cell 168, 326 (2017).
Bluher, S. et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring) 22, 1701–1708 (2014).
Moreno, M. et al. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 10, e0124100 (2015).
Sesti, G. et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 51, 705–713 (2014).
Stengel, A. et al. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity — correlation with body mass index. Peptides 39, 125–130 (2013).
Duan, H. et al. Anti-diabetic activity of recombinant irisin in STZ-induced insulin-deficient diabetic mice. Int. J. Biol. Macromol. 84, 457–463 (2016).
Crujeiras, A. B. et al. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 63, 520–531 (2014).
Chen, J. Q. et al. Serum irisin level is higher and related with insulin in acanthosis nigricans-related obesity. Exp. Clin. Endocrinol. Diabetes 124, 203–207 (2016).
Crujeiras, A. B. et al. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am. J. Hum. Biol. 26, 198–207 (2014).
Huth, C. et al. Irisin is more strongly predicted by muscle oxidative potential than adiposity in non-diabetic men. J. Physiol. Biochem. 71, 559–568 (2015).
Reinehr, T., Elfers, C., Lass, N. & Roth, C. L. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J. Clin. Endocrinol. Metab. 100, 2123–2130 (2015).
Qiu, S. et al. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism 65, 825–834 (2016). This is a meta-analysis of several studies that shows that irisin is weakly, but positively, associated with insulin resistance.
Park, K. H. et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 98, 4899–4907 (2013).
Anastasilakis, A. D. et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 99, 3247–3255 (2014).
Choi, Y. K. et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 100, 96–101 (2013).
Liu, J. J. et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 27, 365–369 (2013).
Zhang, C. et al. Lower irisin level in patients with type 2 diabetes mellitus: a case-control study and meta-analysis. J. Diabetes 8, 56–62 (2016).
Erol, O. et al. Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study. J. Matern. Fetal Neonatal Med. 29, 3590–3595 (2016).
Zhao, L. et al. Circulating irisin is lower in gestational diabetes mellitus. Endocr. J. 62, 921–926 (2015).
Huh, J. H., Ahn, S. V., Choi, J. H., Koh, S. B. & Chung, C. H. High serum irisin level as an independent predictor of diabetes mellitus: a longitudinal population-based study. Medicine (Baltimore) 95, e3742 (2016).
Hwang, Y. C., Jeon, W. S., Park, C. Y. & Youn, B. S. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc. Diabetol. 15, 9 (2016).
Yan, B. et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 9, e94235 (2014).
Aronis, K. N. et al. Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. Int. J. Obes. (Lond.) 39, 156–161 (2015).
Emanuele, E. et al. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am. J. Med. 127, 888–890 (2014).
Acknowledgements
C.S.M. is supported by US National Institutes of Health (NIH DK081913). J.M.F-R. is supported by Instituto Salud Carlos III (http://www.isciii.es); Centro de Investigación Biomédica en Red-Fisiopatología de la Obesidad y Nutrición (CIBERobn) (http://www.ciberobn.es); Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR) (www.gencat.cat/agaur/) (2015FI_B00570); and Fondo Europeo de Desarrollo Regional (FEDER).
Author information
Authors and Affiliations
Contributions
N.P. researched data for the article and wrote the manuscript. G.A.T. researched data for the article, wrote the manuscript and edited the article before submission. J.M.F-R., J.Y.H., K.H.P., J.S. and C.S.M. contributed to discussion of the content, wrote parts of the article and reviewed and/or edited the article before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Human studies investigating the association of irisin with obesity, insulin resistance, diabetes mellitus and metabolic syndrome (PDF 566 kb)
Rights and permissions
About this article
Cite this article
Perakakis, N., Triantafyllou, G., Fernández-Real, J. et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 13, 324–337 (2017). https://doi.org/10.1038/nrendo.2016.221
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.221
This article is cited by
-
Irisin attenuates type 1 diabetic cardiomyopathy by anti-ferroptosis via SIRT1-mediated deacetylation of p53
Cardiovascular Diabetology (2024)
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
A case–control study to evaluate irisin levels in patients with type 1 diabetes mellitus
Irish Journal of Medical Science (1971 -) (2024)
-
Hepatoprotective effects of N-acetylcysteine on liver injury by irisin upregulation and oxidative stress reduction in diabetic rats
Egyptian Liver Journal (2023)
-
Chronic voluntary wheel running exercise ameliorates metabolic dysfunction via PGC-1α expression independently of FNDC5/irisin pathway in high fat diet-induced obese mice
The Journal of Physiological Sciences (2023)