Key Points
-
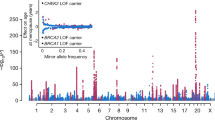
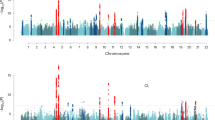
The genetic architecture of pubertal timing and age at menopause involves hundreds of rare, low frequency and common variants, with allelic heterogeneity often apparent at the same loci
-
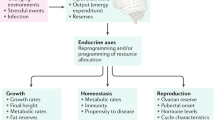
Progress in localizing these variants has shed light on the wide range of biological mechanisms that regulate these traits, including energy homeostasis, gene silencing and DNA repair
-
Population variability in age at menarche and menopause is associated with altered health outcomes in later life, notably earlier timings increase the risk of type 2 diabetes mellitus and cardiovascular disease
-
Mechanisms that link reproductive ageing to metabolic diseases involve both obesity-related pathways and pathways independent of BMI
-
Exposure to sex hormones is probably the overwhelming mechanism linking reproductive ageing to the risk of breast cancer and other hormone-sensitive cancers
Abstract
As age at pubertal onset declines and age at first pregnancy increases, the mechanisms that regulate female reproductive lifespan become increasingly relevant to population health. The timing of menarche and menopause can have profound effects not only on fertility but also on the risk of diseases such as type 2 diabetes mellitus, cardiovascular disease and breast cancer. Genetic studies have identified dozens of highly penetrant rare mutations associated with reproductive disorders, and also ∼175 common genetic variants associated with the timing of puberty or menopause. These findings, alongside other functional studies, have highlighted a diverse range of mechanisms involved in reproductive ageing, implicating core biological processes such as cell cycle regulation and energy homeostasis. The aim of this article is to review the contribution of such genetic findings to our understanding of the molecular regulation of reproductive timing, as well as the biological basis of the epidemiological links between reproductive ageing and disease risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chumlea, W. C. et al. Age at menarche and racial comparisons in US girls. Pediatrics 111, 110–113 (2003).
Parent, A.-S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Remsberg, K. E. et al. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J. Clin. Endocrinol. Metab. 90, 2718–2724 (2005).
Hartge, P. Genetics of reproductive lifespan. Nat. Genet. 41, 637–638 (2009).
Palmert, M. R. & Boepple, P. A. Variation in the timing of puberty: clinical spectrum and genetic investigation. J. Clin. Endocrinol. Metab. 86, 2364–2368 (2001).
Morris, D. H. et al. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am. J. Epidemiol. 175, 998–1005 (2012).
Coulam, C. B., Adamson, S. C. & Annegers, J. F. Incidence of premature ovarian failure. Obstet. Gynecol. 67, 604–606 (1986).
Lambalk, C. B., van Disseldorp, J., de Koning, C. H. & Broekmans, F. J. Testing ovarian reserve to predict age at menopause. Maturitas 63, 280–291 (2009).
Carolan, M. The graying of the obstetric population: implications for the older mother. J. Obstet. Gynecol. Neonatal Nurs. 32, 19–27 (2003).
Fuqua, J. S. Treatment and outcomes of precocious puberty: an update. J. Clin. Endocrinol. Metab. 98, 2198–2207 (2013).
Baams, L., Dubas, J. S., Overbeek, G. & van Aken, M. A. G. Transitions in body and behavior: a meta-analytic study on the relationship between pubertal development and adolescent sexual behavior. J. Adolesc. Health 56, 586–598 (2015).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. bioRxiv http://dx.doi.org/10.1101/014498.
Day, F. R. et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat. Genet. http://dx.doi.org/10.1038/ng.3412.
Day, F. R., Elks, C. E., Murray, A., Ong, K. K. & Perry, J. R. B. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci. Rep. 5, 11208 (2015).
Silveira, L. F. G. & Latronico, A. C. Approach to the patient with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 98, 1781–1788 (2013).
Bianco, S. D. C. & Kaiser, U. B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 5, 569–576 (2009).
Di Pasquale, E. et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J. Clin. Endocrinol. Metab. 91, 1976–1979 (2006).
Bione, S. et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am. J. Hum. Genet. 62, 533–541 (1998).
Pittman, D. L. et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol. Cell 1, 697–705 (1998).
Mandon-Pépin, B. et al. Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur. J. Endocrinol. 158, 107–115 (2008).
Fogli, A. et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am. J. Hum. Genet. 72, 1544–50 (2003).
Pangas, S. A. et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl Acad. Sci. USA 103, 8090–8095 (2006).
Zhao, H. et al. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am. J. Hum. Genet. 82, 1342–1348 (2008).
Nallathambi, J. et al. A novel polyalanine expansion in FOXL2: the first evidence for a recessive form of the blepharophimosis syndrome (BPES) associated with ovarian dysfunction. Hum. Genet. 121, 107–112 (2007).
Ghadami, M. et al. Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR−/− FORKO mice. Mol. Hum. Reprod. 16, 241–250 (2010).
Wang, J., Zhang, W., Jiang, H. & Wu, B.-L. Mutations in HFM1 in recessive primary ovarian insufficiency. N. Engl. J. Med. 370, 972–974 (2014).
Latronico, A. C. et al. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. N. Engl. J. Med. 334, 507–512 (1996).
De Vries, S. S. et al. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev. 13, 523–531 (1999).
Qin, Y. et al. NOBOX homeobox mutation causes premature ovarian failure. Am. J. Hum. Genet. 81, 576–581 (2007).
Lourenço, D. et al. Mutations in NR5A1 associated with ovarian insufficiency. N. Engl. J. Med. 360, 1200–1210 (2009).
Mansouri, M. R. et al. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum. Mol. Genet. 17, 3776–3783 (2008).
Lacombe, A. et al. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am. J. Hum. Genet. 79, 113–119 (2006).
Luoma, P. et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase γ mutations: clinical and molecular genetic study. Lancet 364, 875–882 (2004).
Caburet, S. et al. Mutant cohesin in premature ovarian failure. N. Engl. J. Med. 370, 943–949 (2014).
AlAsiri, S. et al. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J. Clin. Invest. 125, 258–262 (2015).
Wood-Trageser, M. A. et al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am. J. Hum. Genet. 95, 754–762 (2014).
Murray, A. et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet. Med. 16, 19–24 (2014).
Abreu, A. P. et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 368, 2467–2475 (2013).
Teles, M. G. et al. A GPR54-activating mutation in a patient with central precocious puberty. N. Engl. J. Med. 358, 709–715 (2008).
Zhu, J. et al. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 100, E646–E654 (2015).
Perry, J. R. B. et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97 (2014).
Cousminer, D. L. et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 23, 4452–4464 (2014).
Lunetta, K. L. et al. Rare coding variants and X-linked loci associated with age at menarche. Nat. Commun. http://dx.doi.org/10.1038/ncomms8756.
Topaloglu, A. K. et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 41, 354–358 (2009).
Joustra, S. D. et al. The IGSF1 deficiency syndrome: characteristics of male and female patients. J. Clin. Endocrinol. Metab. 98, 4942–4952 (2013).
Zhu, X. et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl Acad. Sci. USA 99, 10293–10298 (2002).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Prentice, P. & Viner, R. M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int. J. Obes. (Lond.) 37, 1036–1043 (2013).
Strobel, A., Issad, T., Camoin, L., Ozata, M. & Strosberg, A. D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18, 213–215 (1998).
Farooqi, I. S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Garrel, G. et al. Unsaturated fatty acids disrupt Smad signaling in gonadotrope cells leading to inhibition of FSHβ gene expression. Endocrinology 155, 592–604 (2014).
Elks, C. E. et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 7, e1000284 (2010).
Schoenaker, D. A., Jackson, C. A., Rowlands, J. V & Mishra, G. D. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int. J. Epidemiol. 43, 1542–1562 (2014).
Tao, X. et al. Body mass index and age at natural menopause: a meta-analysis. Menopause 22, 469–474 (2015).
Layman, L. C. et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N. Engl. J. Med. 337, 607–611 (1997).
Aittomäki, K. et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82, 959–968 (1995).
Sun, Y. et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat. Genet. 44, 1375–1381 (2012).
Cho, S. et al. 9-cis-retinoic acid represses transcription of the gonadotropin-releasing hormone (GnRH) gene via proximal promoter region that is distinct from all-trans-retinoic acid response element. Mol. Brain Res. 87, 214–222 (2001).
Rance, N. E. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30, 111–122 (2009).
Gaytan, F. et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 155, 3088–3097 (2014).
Hughes, I. A. Releasing the brake on puberty. N. Engl. J. Med. 368, 2513–2515 (2013).
Ong, K. K. et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 41, 729–733 (2009).
He, C. et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat. Genet. 41, 724–728 (2009).
Perry, J. R. et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat. Genet. 41, 648–650 (2009).
Sulem, P. et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat. Genet. 41, 734–738 (2009).
Kumar, R. M. et al. Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature 516, 56–61 (2014).
Shyh-Chang, N. & Daley, G. Q. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12, 395–406 (2013).
Nishi, K., Nishi, A., Nagasawa, T. & Ui-Tei, K. Human TNRC6A is an Argonaute-navigator protein for microRNA-mediated gene silencing in the nucleus. RNA 19, 17–35 (2013).
Lomniczi, A. et al. Epigenetic control of female puberty. Nat. Neurosci. 16, 281–289 (2013).
Johnson, J., Canning, J., Kaneko, T., Pru, J. K. & Tilly, J. L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004).
Zou, K. et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 11, 631–636 (2009).
White, Y. A. R. et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 18, 413–421 (2012).
Hu, Y.-C. et al. Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 11, e1005019 (2015).
Tripathi, A., Kumar, K. V. P. & Chaube, S. K. Meiotic cell cycle arrest in mammalian oocytes. J. Cell. Physiol. 223, 592–600 (2010).
Dunlop, C. E., Telfer, E. E. & Anderson, R. A. Ovarian germline stem cells. Stem Cell Res. Ther. 5, 98 (2014).
Nagaoka, S. I., Hassold, T. J. & Hunt, P. A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 (2012).
Henderson, S. A. & Edwards, R. G. Chiasma frequency and maternal age in mammals. Nature 218, 22–28 (1968).
Polani, P. E. & Crolla, J. A. A test of the production line hypothesis of mammalian oogenesis. Hum. Genet. 88, 64–70 (1991).
Koehler, K. E., Hawley, R. S., Sherman, S. & Hassold, T. Recombination and nondisjunction in humans and flies. Hum. Mol. Genet. 5, 1495–1504 (1996).
Rowsey, R., Gruhn, J., Broman, K. W., Hunt, P. A. & Hassold, T. Examining variation in recombination levels in the human female: a test of the production-line hypothesis. Am. J. Hum. Genet. 95, 108–112 (2014).
De Vries, L. et al. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 99, E2129–E2132 (2014).
Stolk, L. et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 44, 260–268 (2012).
Titus, S. et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 5, 172ra21 (2013).
Levy-Lahad, E. & Friedman, E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 96, 11–15 (2007).
Oktay, K., Kim, J. Y., Barad, D. & Babayev, S. N. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J. Clin. Oncol. 28, 240–244 (2010).
Park, J. et al. The MCM8–MCM9 complex promotes RAD51 recruitment at DNA damage sites to facilitate homologous recombination. Mol. Cell. Biol. 33, 1632–1644 (2013).
Lutzmann, M. et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol. Cell 47, 523–534 (2012).
Pierce, S. B. et al. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am. J. Hum. Genet. 92, 614–620 (2013).
Jenkinson, E. M. et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am. J. Hum. Genet. 92, 605–613 (2013).
Pierce, S. B. et al. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl Acad. Sci. USA 108, 6543–6548 (2011).
Lu, C. et al. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum. Mol. Genet. 21, 5039–5047 (2012).
Hoffman, G. E. et al. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J. Histochem. Cytochem. 60, 439–456 (2012).
Oshima, J., Martin, G. & Hisama, F. Werner syndrome. GeneReviews®[online], (2002).
Silva, C. A. et al. Autoimmune primary ovarian insufficiency. Autoimmun. Rev. 13, 427–430 (2014).
Anasti, J. N. et al. Karyotypically normal spontaneous premature ovarian failure: evaluation of association with the class II major histocompatibility complex. J. Clin. Endocrinol. Metab. 78, 722–723 (1994).
Jaroudi, K. A., Arora, M., Sheth, K. V., Sieck, U. V. & Willemsen, W. N. Human leukocyte antigen typing and associated abnormalities in premature ovarian failure. Hum. Reprod. 9, 2006–2009 (1994).
Robb, L. et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 4, 303–308 (1998).
Northup, J., Griffis, K., Hawkins, J., Lockhart, L. & Velagaleti, G. Unusual pseudo dicentric, psu dic (1;19)(q10;q13.42), in a female with premature ovarian failure. Fertil. Steril. 87, 697.e5–697.e8 (2007).
Tong, Z. B. & Nelson, L. M. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology 140, 3720–3726 (1999).
Onland-Moret, N. C. et al. Age at menarche in relation to adult height: the EPIC study. Am. J. Epidemiol. 162, 623–632 (2005).
Xie, H. et al. Homeodomain proteins SIX3 and SIX6 regulate gonadotrope-specific genes during pituitary development. Mol. Endocrinol. 29, 842–855 (2015).
Horikoshi, M. et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 45, 76–82 (2013).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196 (2015).
D'Aloisio, A. A., DeRoo, L. A., Baird, D. D., Weinberg, C. R. & Sandler, D. P. Prenatal and infant exposures and age at menarche. Epidemiology 24, 277–284 (2013).
Andersson, E. A. et al. The birth weight lowering C-allele of rs900400 near LEKR1 and CCNL1 associates with elevated insulin release following an oral glucose challenge. PLoS ONE 6, e27096 (2011).
Elks, C. E. et al. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care 36, 3526–3534 (2013).
Canoy, D. et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 131, 237–244 (2015).
Shuster, L. T., Rhodes, D. J., Gostout, B. S., Grossardt, B. R. & Rocca, W. A. Premature menopause or early menopause: long-term health consequences. Maturitas 65, 161–166 (2010).
Brand, J. S. et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 36, 1012–1019 (2012).
Zhu, H. et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 (2011).
Purwana, I. et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 63, 4197–4205 (2014).
Dupuis, J. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42, 105–116 (2010).
Anderson, K. N., Schwab, R. B. & Martinez, M. E. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat. 144, 1–10 (2014).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Perry, J., Murray, A., Day, F. et al. Molecular insights into the aetiology of female reproductive ageing. Nat Rev Endocrinol 11, 725–734 (2015). https://doi.org/10.1038/nrendo.2015.167
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.167
This article is cited by
-
Mendelian randomization identifies circulating proteins as biomarkers for age at menarche and age at natural menopause
Communications Biology (2024)
-
Sex differences in systemic metabolites at four life stages: cohort study with repeated metabolomics
BMC Medicine (2021)
-
Polygenic interactions with adiposity rebound in the prediction of thelarche
Pediatric Research (2021)
-
Elucidating the genetic architecture of reproductive ageing in the Japanese population
Nature Communications (2018)
-
Complex genetics of female fertility
npj Genomic Medicine (2018)