Abstract
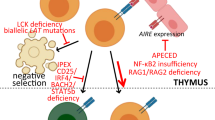
The autoimmune regulator (AIRE) gene encodes a transcription factor involved in the presentation of tissue-restricted antigens during T-cell development in the thymus. Mutations of this gene lead to type 1 autoimmune polyglandular syndrome (APS-1), also termed autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome, which is characterized by the clinical presentation of at least two of a triad of underlying disorders: Addison disease, hypoparathyroidism and chronic mucocutaneous candidiasis. This Review describes the process of positive and negative selection of developing T cells in the thymus and the role of AIRE as a regulator of peripheral antigen presentation. Furthermore, it addresses how mutations of this gene lead to the failure to eliminate autoreactive T cells, which can lead to clinical autoimmune syndromes.
Key Points
-
More than 60 mutations of the autoimmune regulator (AIRE) gene are associated with the development of type 1 autoimmune polyglandular syndrome (APS-1) or autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome
-
AIRE plays an important part in shaping the T-cell repertoire through its role in negative selection of autoreactive T cells
-
In addition, mutations of AIRE can directly, by affecting the deletion of autoreactive T cells in the thymus, and indirectly, by promoting anti-interferon antibodies, affect other aspects of immune responses
-
The clinical manifestations associated with APS-1 classically involve mucocutaneous candidiasis, hypoparathyroidism and adrenal insufficiency, but can vary in scope and timing
-
Production of autoantibodies against cytokines, including interferon α, interleukin 17A and interleukin 22, in patients with APS-1 may be responsible for the development of mucocutaneous candidiasis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bubanovic, I. V. Crossroads of extrathymic lymphocytes maturation pathways. Med. Hypotheses 61, 235–239 (2003).
Pantelouris, E. M. Absence of thymus in a mouse mutant. Nature 217, 370–371 (1968).
Cunliffe, V. T., Furley, A. J. & Keenan, D. Complete rescue of the nude mutant phenotype by a wild-type Foxn1 transgene. Mamm. Genome 13, 245–252 (2002).
Lind, E. F., Prockop, S. E., Porritt, H. E. & Petrie, H. T. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J. Exp. Med. 194, 127–134 (2001).
Pearse, M. et al. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc. Natl Acad. Sci. USA 86, 1614–1618 (1989).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Jameson, S. C., Hogquist, K. A. & Bevan, M. J. Positive selection of thymocytes. Annu. Rev. Immunol. 13, 93–126 (1995).
Kisielow, P., Teh, H. S., Blüthmann, H. & von Boehmer, H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 335, 730–733 (1988).
Scollay, R. G., Butcher, E. C. & Weissman, I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 10, 210–218 (1980).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
Silva-Santos, B., Pennington, D. J. & Hayday, A. C. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science 307, 925–928 (2005).
Chin, R. K. et al. Lymphotoxin pathway directs thymic Aire expression. Nat. Immunol. 4, 1121–1127 (2003).
Martins, V. C., Boehm, T. & Bleul, C. C. Ltβr signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J. Immunol. 181, 400–407 (2008).
Venanzi, E. S., Melamed, R., Mathis, D. & Benoist, C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc. Natl Acad. Sci. USA 105, 15860–15865 (2008).
Derbinski, J. et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45 (2005).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
Abramson, J., Giraud, M., Benoist, C. & Mathis, D. Aire's partners in the molecular control of immunological tolerance. Cell 140, 123–135 (2010).
Guerau-de-Arellano, M., Mathis, D. & Benoist, C. Transcriptional impact of Aire varies with cell type. Proc. Natl Acad. Sci. USA 105, 14011–14016 (2008).
Ferguson, B. J. et al. AIRE's CARD revealed, a new structure for central tolerance provokes transcriptional plasticity. J. Biol. Chem. 283, 1723–1731 (2008).
Mathis, D. & Benoist, C. A decade of AIRE. Nat. Rev. Immunol. 7, 645–650 (2007).
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001).
Heino, M. et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur. J. Immunol. 30, 1884–1893 (2000).
Kogawa, K. et al. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol. Lett. 80, 195–198 (2002).
Su, M. A. et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J. Clin. Invest. 118, 1712–1726 (2008).
Lee, J. W. et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 8, 181–190 (2007).
Gardner, J. M. et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843-7 (2008).
Poliani, P. L. et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am. J. Pathol. 176, 1104–1112 (2010).
Ikegami, H. Animal models of autoimmune polyglandular syndrome. Endocrinol. Metab. Clin. North Am. 31, 431–439 (2002).
Ramsey, C. et al. Increased antigen presenting cell-mediated T cell activation in mice and patients without the autoimmune regulator. Eur. J. Immunol. 36, 305–317 (2006).
Gotter, J. & Kyewski, B. Regulating self-tolerance by deregulating gene expression. Curr. Opin. Immunol. 16, 741–745 (2004).
Johnnidis, J. B. et al. Chromosomal clustering of genes controlled by the aire transcription factor. Proc. Natl Acad. Sci. USA 102, 7233–7238 (2005).
Jiang, W., Anderson, M. S., Bronson, R., Mathis, D. & Benoist, C. Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202, 805–815 (2005).
Fan, Y. et al. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 28, 2812–2824 (2009).
Babaya, N. et al. A new model of insulin-deficient diabetes: male NOD mice with a single copy of Ins1 and no Ins2. Diabetologia 49, 1222–1228 (2006).
Jaeckel, E., Lipes, M. A. & von Boehmer, H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat. Immunol. 5, 1028–1035 (2004).
Kuroda, N. et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J. Immunol. 174, 1862–1870 (2005).
Niki, S. et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J. Clin. Invest. 116, 1292–1301 (2006).
Björses, P. et al. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am. J. Hum. Genet. 66, 378–392 (2000).
Wolff, A. S. et al. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J. Clin. Endocrinol. Metab. 92, 595–603 (2007).
Piirilä, H., Väliaho, J. & Vihinen, M. Immunodeficiency mutation databases (IDbases). Hum. Mutat. 27, 1200–1208 (2006), [online] (2010).
Ahonen, P. Autoimmune polyendocrinopathy-candidosis--ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin. Genet. 27, 535–542 (1985).
Zlotogora, J. & Shapiro, M. S. Polyglandular autoimmune syndrome type I among Iranian Jews. J. Med. Genet. 29, 824–826 (1992).
Aaltonen, J., Björses, P., Sandkuijl, L., Perheentupa, J. & Peltonen, L. An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat. Genet. 8, 83–87 (1994).
Björses, P. et al. Genetic homogeneity of autoimmune polyglandular disease type I. Am. J. Hum. Genet. 59, 879–886 (1996).
Aaltonen, J. et al. High-resolution physical and transcriptional mapping of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy locus on chromosome 21q22.3 by FISH. Genome Res. 7, 820–829 (1997).
Kudoh, J. et al. Localization of 16 exons to a 450-kb region involved in the autoimmune polyglandular disease type I (APECED) on human chromosome 21q22.3. DNA Res. 4, 45–52 (1997).
Nagamine, K. et al. Positional cloning of the APECED gene. Nat. Genet. 17, 393–398 (1997).
Vogel, A. et al. Autoimmune regulator AIRE: evidence for genetic differences between autoimmune hepatitis and hepatitis as part of the autoimmune polyglandular syndrome type 1. Hepatology 33, 1047–1052 (2001).
Peterson, P., Pitkänen, J., Sillanpää, N. & Krohn, K. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED): a model disease to study molecular aspects of endocrine autoimmunity. Clin. Exp. Immunol. 135, 348–357 (2004).
Pearce, S. H. et al. A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am. J. Hum. Genet. 63, 1675–1684 (1998).
Heino, M. et al. Mutation analyses of North American APS-1 patients. Hum. Mutat. 13, 69–74 (1999).
[No authors listed] An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 17, 399–403 (1997).
Cervato, S. et al. Evaluation of the autoimmune regulator (AIRE) gene mutations in a cohort of Italian patients with autoimmune-polyendocrinopathy-candidiasis-ectodermal-dystrophy (APECED) and in their relatives. Clin. Endocrinol. (Oxf.) 70, 421–428 (2009).
Cihakova, D. et al. Novel AIRE mutations and P450 cytochrome autoantibodies in Central and Eastern European patients with APECED. Hum. Mutat. 18, 225–232 (2001).
Heino, M. et al. APECED mutations in the autoimmune regulator (AIRE) gene. Hum. Mutat. 18, 205–211 (2001).
Husebye, E. S., Perheentupa, J., Rautemaa, R. & Kämpe, O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J. Intern. Med. 265, 514–529 (2009).
Podkrajsek, K. T. et al. Detection of a complete autoimmune regulator gene deletion and two additional novel mutations in a cohort of patients with atypical phenotypic variants of autoimmune polyglandular syndrome type 1. Eur. J. Endocrinol. 159, 633–639 (2008).
Buzi, F. et al. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome: time to review diagnostic criteria? J. Clin. Endocrinol. Metab. 88, 3146–3148 (2003).
Blizzard, R. M. & Kyle, M. Studies of the adrenal antigens and antibodies in Addison's disease. J. Clin. Invest. 42, 1653–1660 (1963).
Hung, W., Migeon, C. J. & Parrott, R. H. A possible autoimmune basis for Addison's disease in three siblings, one with idiopathic hypoparathyroidism, pernicious anemia and superficial moniliasis. N. Engl. J. Med. 269, 658–663 (1963).
Kogut, M. D. & Brinegar, C. H. Jr. Addison's disease and diabetes mellitus. J. Pediatr. 81, 307–311 (1972).
Alimohammadi, M. et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N. Engl. J. Med. 358, 1018–1028 (2008).
Meager, A. et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3, e289 (2006).
Kisand, K. et al. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood 112, 2657–2666 (2008).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Kisand, K. et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010).
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Rautemaa, R., Hietanen, J., Niissalo, S., Pirinen, S. & Perheentupa, J. Oral and oesophageal squamous cell carcinoma—a complication or component of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, APS-I). Oral Oncol. 43, 607–613 (2007).
Ward, L. et al. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J. Clin. Endocrinol. Metab. 84, 844–852 (1999).
Padeh, S., Theodor, R., Jonas, A. & Passwell, J. H. Severe malabsorption in autoimmune polyendocrinopathy-candidosis-ectodermal dystrophy syndrome successfully treated with immunosuppression. Arch. Dis. Child. 76, 532–534 (1997).
Dhodapkar, M. V., Lust, J. A. & Phyliky, R. L. T-cell large granular lymphocytic leukemia and pure red cell aplasia in a patient with type I autoimmune polyendocrinopathy: response to immunosuppressive therapy. Mayo Clin. Proc. 69, 1085–1088 (1994).
Ulinski, T. et al. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome with renal failure: impact of posttransplant immunosuppression on disease activity. J. Clin. Endocrinol. Metab. 91, 192–195 (2006).
Ströbel, P. et al. Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1). J. Pathol. 211, 563–571 (2007).
Evoli, A. et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology 59, 1844–1850 (2002).
Cetani, F. et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 86, 4747–4752 (2001).
Björses, P., Aaltonen, J., Horelli-Kuitunen, N., Yaspo, M. L. & Peltonen, L. Gene defect behind APECED: a new clue to autoimmunity. Hum. Mol. Genet. 7, 1547–1553 (1998).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Ryan, K. R. et al. CD4+CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J. Allergy Clin. Immunol. 116, 1158–1159 (2005).
Kekäläinen, E. et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Immunol. 178, 1208–1215 (2007).
Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C. C. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4, 350–354 (2003).
Liston, A. et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl Acad. Sci. USA 105, 11903–11908 (2008).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Gebre-Medhin, G. et al. Cytochrome P450IA2 and aromatic L-amino acid decarboxylase are hepatic autoantigens in autoimmune polyendocrine syndrome type I. FEBS Lett. 412, 439–445 (1997).
Tuomi, T. et al. Antibodies to glutamic acid decarboxylase and insulin-dependent diabetes in patients with autoimmune polyendocrine syndrome type I. J. Clin. Endocrinol. Metab. 81, 1488–1494 (1996).
Söderbergh, A. et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J. Clin. Endocrinol. Metab. 89, 557–562 (2004).
Ekwall, O. et al. Identification of tryptophan hydroxylase as an intestinal autoantigen. Lancet 352, 279–283 (1998).
Krohn, K., Uibo, R., Aavik, E., Peterson, P. & Savilahti, K. Identification by molecular cloning of an autoantigen associated with Addison's disease as steroid 17 alpha-hydroxylase. Lancet 339, 770–773 (1992).
Hubert, F. X. et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J. Immunol. 182, 3902–3918 (2009).
Author information
Authors and Affiliations
Contributions
E. M. Akirav and K. C. Herold researched the data for the article. All authors provided a substantial contribution to discussions of the content. E. M. Akirav and K. C. Herold wrote the article, and all authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Akirav, E., Ruddle, N. & Herold, K. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol 7, 25–33 (2011). https://doi.org/10.1038/nrendo.2010.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.200
This article is cited by
-
Regulation of cellular gene expression by nanomaterials
Nano Convergence (2018)
-
A new mutation site in the AIRE gene causes autoimmune polyendocrine syndrome type 1
Immunogenetics (2017)
-
Cerebral toxoplasmosis in a patient with myasthenia gravis and thymoma with immunodeficiency/Good’s syndrome: a case report
BMC Infectious Diseases (2016)
-
Insight into normal thymic activity by assessment of peripheral blood samples
Immunologic Research (2015)
-
Autoimmune Addison disease: pathophysiology and genetic complexity
Nature Reviews Endocrinology (2012)