Abstract
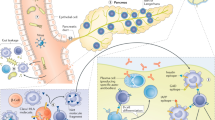
The prevailing concentration of blood glucose is a result of the integrated regulation of insulin secretion and insulin action. Nevertheless, the classic stereotypes of diabetes are dichotomous: type 1 diabetes mellitus (T1DM) is attributed to impaired insulin secretion, and type 2 diabetes mellitus (T2DM) is primarily attributed to impaired insulin action (insulin resistance). The available evidence indicates that this view is overly simplistic. Impaired insulin secretion (β-cell dysfunction) is also a feature of T2DM, and insulin resistance is also a risk factor for the development of T1DM. Moreover, with the increasing incidence of T2DM and T1DM in both developed and developing countries, attributed to environmental factors, the existence of 'hybrid' diabetes types that have clinical and pathogenetic features of both conditions is becoming clearly evident. A common thread across the spectrum of diabetes might be the activation of innate immunological and inflammatory pathways by a proinflammatory environment, which leads to β-cell dysfunction in T2DM, insulin resistance in both T2DM and T1DM, and enhanced adaptive immunity that kills β cells in T1DM. Embracing a holistic view of the diabetes syndrome will help us to understand the environmental basis for the epidemic of diabetes and improve preventative strategies.
Key Points
-
The incidences of both type 1 and type 2 diabetes mellitus have increased over the past half century in the developed world
-
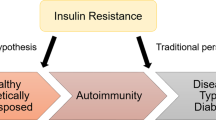
Insulin deficiency and insulin resistance occur in both type 1 and type 2 diabetes
-
Environmental factors, including high-calorie and proinflammatory diets, sedentary lifestyles in sanitized indoor environments and vitamin D insufficiency amongst others, have contributed to the diabetes epidemic
-
Activation of innate immune responses by factors in the modern environment, in association with weight gain, might be a common mechanism that promotes all forms of diabetes
-
Clinicians and scientists must advocate public health policies that target the obesity–diabetes epidemic
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care 32 (Suppl. 1), S62–S67 (2009).
Harrison, L. C. Risk assessment, prediction and prevention of type 1 diabetes. Pediatr. Diabetes 2, 71–82 (2001).
Wenzlau, J. M. et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl Acad. Sci. USA 104, 17040–17045 (2007).
Eisenbarth, G. S. & Pugliese, A. in Type 1 Diabetes: Molecular, Cellular and Clinical Immunology (ed. Eisenbarth, G. S.) 2403–2407 (The Barbara Davis Center for Childhood Diabetes, Denver, 2007).
Florez, J. C. Clinical review: the genetics of type 2 diabetes: a realistic appraisal in 2008. J. Clin. Endocrinol. Metab. 93, 4633–4642 (2008).
Fourlanos, S. et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 48, 2206–2212 (2005).
Gale, E. A. Declassifying diabetes. Diabetologia 49, 1989–1995 (2006).
Wilkin, T. J. Diabetes: 1 and 2, or one and the same? Progress with the accelerator hypothesis. Pediatr. Diabetes 9, 23–32 (2008).
Fox, C. S. et al. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation 113, 2914–2918 (2006).
Gale, E. A. The rise of childhood type 1 diabetes in the 20th century. Diabetes 51, 3353–3361 (2002).
Fourlanos, S. et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care 31, 1546–1549 (2008).
Bruining, G. J. Association between infant growth before onset of juvenile type-1 diabetes and autoantibodies to IA-2. Netherlands Kolibrie study group of childhood diabetes. Lancet 356, 655–656 (2000).
Hypponen, E. et al. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 22, 1961–1965 (1999).
Couper, J. J. et al. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care 32, 94–99 (2009).
Fourlanos, S., Narendran, P., Byrnes, G. B., Colman, P. G. & Harrison, L. C. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 47, 1661–1667 (2004).
Mrena, S. et al. Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care 29, 662–667 (2006).
Xu, P., Cuthbertson, D., Greenbaum, C., Palmer, J. P. & Krischer, J. P. Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 30, 2314–2320 (2007).
Bingley, P. J., Mahon, J. L. & Gale, E. A. Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 31, 146–150 (2008).
Prentice, A. & Jebb, S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr. Rev. 62, S98–S104 (2004).
Esposito, K. et al. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 78, 1135–1140 (2003).
Qi, L. et al. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 29, 207–211 (2006).
Odegaard, A. O. & Pereira, M. A. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr. Rev. 64, 364–372 (2006).
Elliott, S. S., Keim, N. L., Stern, J. S., Teff, K. & Havel, P. J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 76, 911–922 (2002).
Yamagishi, S., Ueda, S. & Okuda, S. Food-derived advanced glycation end products (AGEs): a novel therapeutic target for various disorders. Curr. Pharm. Des. 13, 2832–2836 (2007).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989).
Pozzilli, P., Signore, A., Williams, A. J. & Beales, P. E. NOD mouse colonies around the world—recent facts and figures. Immunol. Today 14, 193–196 (1993).
Funda, D. P., Fundova, P. & Harrison, L. C. Microflora-dependency of selected diabetes-preventive diets: germ-free and ex-germ-free monocolonized NOD mice as models for studying environmental factors in type 1 diabetes [abstract MS-11.4]. In Proc. 13th Int. Cong. Immunol. 16 (Brazilian Society for Immunology, Rio de Janeiro, Brazil, 2007).
Harrison, L. C. et al. Type 1 diabetes: lessons for other autoimmune diseases? J. Autoimmun. 31, 306–310 (2008).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Creely, S. J. et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 292, E740–E747 (2007).
Nowson, C. A. & Margerison, C. Vitamin D intake and vitamin D status of Australians. Med. J. Aust. 177, 149–152 (2002).
Holick, M. F. & Chen, T. C. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 87, 1080S–1086S (2008).
Hypponen, E., Laara, E., Reunanen, A., Jarvelin, M. R. & Virtanen, S. M. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358, 1500–1503 (2001).
Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 69, 842–856 (1999).
[No authors listed] Vitamin D supplementation in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia 42, 51–54 (1999).
Stene, L. C., Joner, G. & Norwegian Childhood Diabetes Study Group. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am. J. Clin. Nutr. 78, 1128–1134 (2003).
Scragg, R., Sowers, M. & Bell, C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 27, 2813–2818 (2004).
Borissova, A. M., Tankova, T., Kirilov, G., Dakovska, L. & Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 57, 258–261 (2003).
Inomata, S., Kadowaki, S., Yamatani, T., Fukase, M. & Fujita, T. Effect of 1α (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. Res. 1, 187–192 (1986).
Chiu, K. C., Chu, A., Go, V. L. & Saad, M. F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 79, 820–825 (2004).
Pittas, A. G., Harris, S. S., Stark, P. C. & Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 30, 980–986 (2007).
Westerterp-Plantenga, M. S., van Marken Lichtenbelt, W. D., Cilissen, C. & Top, S. Energy metabolism in women during short exposure to the thermoneutral zone. Physiol. Behav. 75, 227–235 (2002).
Tasali, E., Mokhlesi, B. & Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133, 496–506 (2008).
Bonnet, M. H. & Arand, D. L. We are chronically sleep-deprived. Sleep 18, 908–911 (1995).
Agras, W. S., Hammer, L. D., McNicholas, F. & Kraemer, H. C. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J. Pediatr. 145, 20–25 (2004).
Hasler, G. et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 27, 661–666 (2004).
Ayas, N. T. et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26, 380–384 (2003).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Pickup, J. C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27, 813–823 (2004).
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Hevener, A. L. et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 117, 1658–1669 (2007).
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Patsouris, D. et al. Ablation of CD11-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell. Metab. 8, 301–309 (2008).
Laybutt, D. R. et al. Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50, 752–763 (2007).
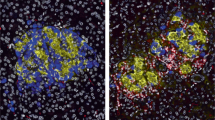
Ehses, J. A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007).
Hutchings, P. et al. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature 348, 639–642 (1990).
Devaraj, S. et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55, 774–779 (2006).
Wang, X. et al. Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J. Immunol. 180, 1929–1937 (2008).
Ebstein, W. Zur therapie des diabetes mellitus, insbesondere uber die anwendeng der salicylauren natron bei demselben [German]. Berl. Klin. Wochenschr. 13, 337–340 (1876).
Williamson, R. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br. Med. J. 1, 760–762 (1901).
Hundal, R. S. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 109, 1321–1326 (2002).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45, 881–885 (1996).
Kiortsis, D. N., Mavridis, A. K., Vasakos, S., Nikas, S. N. & Drosos, A. A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 64, 765–766 (2005).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Stender, S., Dyerberg, J. & Astrup, A. High levels of industrially produced trans fat in popular fast foods. N. Engl. J. Med. 354, 1650–1652 (2006).
Acknowledgements
This work was funded by Program (516700) and Infrastructure (361646) grants from the National Health and Medical Research Council of Australia (NHMRC), a Victorian State Government Operational Infrastructure Support Grant, the Diabetes Australia Research Trust (DART) and the Royal Australian College of Physicians Research Foundation. J. M. Wentworth is a Doherty Fellow, and L. C. Harrison is a Senior Principal Research Fellow, of the NHMRC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wentworth, J., Fourlanos, S. & Harrison, L. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol 5, 483–489 (2009). https://doi.org/10.1038/nrendo.2009.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.149
This article is cited by
-
Investigation of serum level relationship anti-glutamic acid decarboxylase antibody and inflammatory cytokines (IL1-β, IL-6) with vitamins D in type 2 diabetes
Journal of Diabetes & Metabolic Disorders (2022)
-
The Interconnection Between Immuno-Metabolism, Diabetes, and CKD
Current Diabetes Reports (2019)
-
An overview of in vitro and in vivo glycation of albumin: a potential disease marker in diabetes mellitus
Glycoconjugate Journal (2017)
-
Neues zum „late autoimmune diabetes of the adult“ in Europa
Der Diabetologe (2013)
-
AGE restriction in diabetes mellitus: a paradigm shift
Nature Reviews Endocrinology (2011)