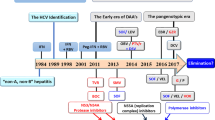
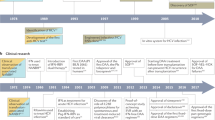
Abstract
Hepatitis C virus (HCV) is a hepatotropic RNA virus that causes progressive liver damage, which might result in liver cirrhosis and hepatocellular carcinoma. Globally, between 64 and 103 million people are chronically infected. Major risk factors for this blood-borne virus infection are unsafe injection drug use and unsterile medical procedures (iatrogenic infections) in countries with high HCV prevalence. Diagnostic procedures include serum HCV antibody testing, HCV RNA measurement, viral genotype and subtype determination and, lately, assessment of resistance-associated substitutions. Various direct-acting antiviral agents (DAAs) have become available, which target three proteins involved in crucial steps of the HCV life cycle: the NS3/4A protease, the NS5A protein and the RNA-dependent RNA polymerase NS5B protein. Combination of two or three of these DAAs can cure (defined as a sustained virological response 12 weeks after treatment) HCV infection in >90% of patients, including populations that have been difficult to treat in the past. As long as a prophylactic vaccine is not available, the HCV pandemic has to be controlled by treatment-as-prevention strategies, effective screening programmes and global access to treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stanaway, J. D. et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388, 1081–1088 (2016).
Gower, E., Estes, C., Blach, S., Razavi-Shearer, K. & Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 61, S45–S57 (2014).
Mohd Hanafiah, K., Groeger, J., Flaxman, A. D. & Wiersma, S. T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57, 1333–1342 (2013).
Lohmann, V. et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285, 110–113 (1999). This paper establishes the HCV replicon system, which is a methodological breakthrough for drug development in HCV infection.
Hoofnagle, J. H. et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 315, 1575–1578 (1986). This is the first study to use IFN in the treatment of hepatitis C before HCV was discovered when the disease was still called non-A, non-B hepatitis.
Lamarre, D. et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426, 186–189 (2003). This is the first study to successfully use and provide proof of concept for a NS3/4A protease inhibitor as the first DAA for HCV infection.
Wakita, T. et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11, 791–796 (2005). This study establishes an in vitro HCV infection in tissue culture.
Lok, A. S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012). This study provides proof of concept that a combination of different classes of DAAs without IFN can cure chronic HCV infection.
Choo, Q. L. et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 (1989). This paper was the first to discover HCV.
Manns, M. P. & von Hahn, T. Novel therapies for hepatitis C — one pill fits all? Nat. Rev. Drug Discov. 12, 595–610 (2013).
Manns, M. P. et al. Long-term clearance of hepatitis C virus following interferon alpha-2b or peginterferon alpha-2b, alone or in combination with ribavirin. J. Viral Hepat. 20, 524–529 (2013).
Swain, M. G. et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 139, 1593–1601 (2010).
Younossi, Z. M. et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 12, 1349–1359.e13 (2014).
Pawlotsky, J. M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 151, 70–86 (2016). This review defines and explains the relevance, diagnosis and management of drug resistance and RASs of DAAs.
The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2, 161–176 (2017).
Nerrienet, E. et al. Hepatitis C virus infection in Cameroon: a cohort-effect. J. Med. Virol. 76, 208–214 (2005).
Njouom, R. et al. Phylogeography, risk factors and genetic history of hepatitis C virus in Gabon, Central Africa. PLoS ONE 7, e42002 (2012).
Sharvadze, L., Nelson, K. E., Imnadze, P., Karchava, M. & Tsertsvadze, T. Prevalence of HCV and genotypes distribution in general population of Georgia. Georgian Med. News 165, 71–77 (2008).
Baatarkhuu, O. et al. Prevalence and genotype distribution of hepatitis C virus among apparently healthy individuals in Mongolia: a population-based nationwide study. Liver Int. 28, 1389–1395 (2008).
Qureshi, H., Bile, K. M., Jooma, R., Alam, S. E. & Afridi, H. U. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East. Mediterr. Health J. 16, S15–S23 (2010).
Ruzibakiev, R. et al. Risk factors and seroprevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection in Uzbekistan. Intervirology 44, 327–332 (2001).
Arafa, N. et al. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J. Hepatol. 43, 418–424 (2005).
Ministry of Health and Population, El-Zanaty and Associates & ICF International. Egypt health issues survey 2015. DHS Programhttps://dhsprogram.com/pubs/pdf/FR313/FR313.pdf (2015).
Razavi, H. et al. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J. Viral Hepat. 21 (Suppl. 1), 34–59 (2014).
Hatzakis, A. et al. The present and future disease burden of hepatitis C virus (HCV) infections with today's treatment paradigm — volume 2. J. Viral Hepat. 22 (Suppl. 1), 26–45 (2015).
Alter, M. J., Kuhnert, W. L., Finelli, L. & Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 52, 1–16 (2003).
Schmidt, A. J. et al. Prevalence of hepatitis C in a Swiss sample of men who have sex with men: whom to screen for HCV infection? BMC Public Health 14, 3 (2014).
Dalgard, O. et al. Risk factors for hepatitis C among injecting drug users in Oslo. Tidsskr. Nor. Laegeforen. 129, 101–104 (in Norwegian) (2009).
Duberg, A., Janzon, R., Back, E., Ekdahl, K. & Blaxhult, A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 13, 18882 (2008).
Mann, A. G. et al. Diagnoses of, and deaths from, severe liver disease due to hepatitis C in England between 2000 and 2005 estimated using multiple data sources. Epidemiol. Infect. 137, 513–518 (2009).
Public Health Agency of Canada. A study to characterize the epidemiology of hepatitis C infection in Canada, 2002. Public Health Agency Canadahttp://publications.gc.ca/collections/collection_2009/aspc-phac/HP40-31-2008E.pdf (2008).
[No authors listed.] Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 47, 1–39 (1998).
U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Forcehttp://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm (2013).
Osaki, Y. & Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol. Res. 45, 59–74 (2015).
Alfaleh, F. Z. et al. Strategies to manage hepatitis C virus infection disease burden — volume 3. J. Viral Hepat. 22 (Suppl. 4), 42–65 (2015).
Gane, E. et al. Strategies to manage hepatitis C virus (HCV) infection disease burden — volume 2. J. Viral Hepat. 22 (Suppl. 1), 46–73 (2015).
Wedemeyer, H. et al. Strategies to manage hepatitis C virus (HCV) disease burden. J. Viral Hepat. 21 (Suppl. 1), 60–89 (2014).
Negro, F. et al. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 149, 1345–1360 (2015).
van der Meer, A. J. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593 (2012). This study provides proof that cure of hepatitis C can reduce liver and overall mortality.
Burbelo, P. D. et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J. Virol. 86, 6171–6178 (2012).
Kapoor, A. et al. Characterization of a canine homolog of hepatitis C virus. Proc. Natl Acad. Sci. USA 108, 11608–11613 (2011).
Kapoor, A. et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio 4, e00216-13 (2013).
Lyons, S. et al. Nonprimate hepaciviruses in domestic horses, United Kingdom. Emerg. Infect. Dis. 18, 1976–1982 (2012).
Simmonds, P. The origin of hepatitis C virus. Curr. Top. Microbiol. Immunol. 369, 1–15 (2013).
Penin, F., Dubuisson, J., Rey, F. A., Moradpour, D. & Pawlotsky, J. M. Structural biology of hepatitis C virus. Hepatology 39, 5–19 (2004).
Andre, P. et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 76, 6919–6928 (2002).
Zeisel, M. B., Felmlee, D. J. & Baumert, T. F. Hepatitis C virus entry. Curr. Top. Microbiol. Immunol. 369, 87–112 (2013).
Timpe, J. M. et al. Hepatitis C virus cell–cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47, 17–24 (2008).
Honda, M., Beard, M. R., Ping, L. H. & Lemon, S. M. A phylogenetically conserved stem-loop structure at the 5' border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J. Virol. 73, 1165–1174 (1999).
Niepmann, M. Hepatitis C virus RNA translation. Curr. Top. Microbiol. Immunol. 369, 143–166 (2013).
Moradpour, D. & Penin, F. Hepatitis C virus proteins: from structure to function. Curr. Top. Microbiol. Immunol. 369, 113–142 (2013).
Lohmann, V. Hepatitis C virus RNA replication. Curr. Top. Microbiol. Immunol. 369, 167–198 (2013).
Scheel, T. K. & Rice, C. M. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 19, 837–849 (2013).
Lindenbach, B. D. Virion assembly and release. Curr. Top. Microbiol. Immunol. 369, 199–218 (2013).
Manns, M. P. & Cornberg, M. Sofosbuvir: the final nail in the coffin for hepatitis C? Lancet Infect. Dis. 13, 378–379 (2013).
Janssen, H. L. et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 (2013).
Pawlotsky, J. M. Hepatitis C virus population dynamics during infection. Curr. Top. Microbiol. Immunol. 299, 261–284 (2006).
Khakoo, S. I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Yu, M. Y. et al. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc. Natl Acad. Sci. USA 101, 7705–7710 (2004).
Pestka, J. M. et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl Acad. Sci. USA 104, 6025–6030 (2007).
Gerlach, J. T. et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology 117, 933–941 (1999).
Schulze Zur Wiesch, J. et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 209, 61–75 (2012).
Day, C. L. et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 112, 831–842 (2003).
Grakoui, A. et al. HCV persistence and immune evasion in the absence of memory T cell help. Science 302, 659–662 (2003).
Bowen, D. G. & Walker, C. M. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 201, 1709–1714 (2005).
Heim, M. H. & Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 61, S14–S25 (2014).
Hengst, J. et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 46, 2204–2210 (2016).
Rehermann, B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat. Med. 19, 859–868 (2013).
Radziewicz, H. et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81, 2545–2553 (2007).
Kroy, D. C. et al. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology 146, 550–561 (2014).
Park, S. H. & Rehermann, B. Immune responses to HCV and other hepatitis viruses. Immunity 40, 13–24 (2014).
Moradpour, D., Grakoui, A. & Manns, M. P. Future landscape of hepatitis C research — basic, translational and clinical perspectives. J. Hepatol. 65, S143–S155 (2016). This review describes the future landscape of HCV research.
Yamane, D., McGivern, D. R., Masaki, T. & Lemon, S. M. Liver injury and disease pathogenesis in chronic hepatitis C. Curr. Top. Microbiol. Immunol. 369, 263–288 (2013).
Neumann-Haefelin, C. & Thimme, R. Adaptive immune responses in hepatitis C virus infection. Curr. Top. Microbiol. Immunol. 369, 243–262 (2013).
Nishitsuji, H. et al. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J. Virol. 87, 8169–8178 (2013).
Bonilla, N. et al. Interferon gamma-secreting HCV-specific CD8+ T cells in the liver of patients with chronic C hepatitis: relation to liver fibrosis — ANRS HC EP07 study. J. Viral Hepat. 13, 474–481 (2006).
Franceschini, D. et al. Polyfunctional type-1, -2, and -17 CD8+ T cell responses to apoptotic self-antigens correlate with the chronic evolution of hepatitis C virus infection. PLoS Pathog. 8, e1002759 (2012).
El-Serag, H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273.e1 (2012).
Rusyn, I. & Lemon, S. M. Mechanisms of HCV-induced liver cancer: what did we learn from in vitro and animal studies?. Cancer Lett. 345, 210–215 (2014).
Boyer, T. D., Manns, M. P. & Sanyal, A. J. (eds) Zakim and Boyer's Hepatology, A Textbook of Liver Disease (Elsevier Saunders Philadelphia, 2012).
Chevaliez, S., Rodriguez, C. & Pawlotsky, J. M. New virologic tools for management of chronic hepatitis B and C. Gastroenterology 142, 1303–1313.e1 (2012).
Takaki, A. et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6, 578–582 (2000).
Lee, S. R. et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J. Virol. Methods 172, 27–31 (2011).
Kania, D. et al. Combining rapid diagnostic tests and dried blood spot assays for point-of-care testing of human immunodeficiency virus, hepatitis B and hepatitis C infections in Burkina Faso, West Africa. Clin. Microbiol. Infect. 19, E533–E541 (2013).
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J. Hepatol. 63, 199–236 (2015).
Chevaliez, S., Bouvier-Alias, M., Brillet, R. & Pawlotsky, J. M. Hepatitis C virus (HCV) genotype 1 subtype identification in new HCV drug development and future clinical practice. PLoS ONE 4, e8209 (2009).
Bouvier-Alias, M. et al. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36, 211–218 (2002).
Chevaliez, S., Soulier, A., Poiteau, L., Bouvier-Alias, M. & Pawlotsky, J. M. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. J. Clin. Virol. 61, 145–148 (2014).
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. FDAhttp://www.fda.gov/downloads/Drugs/Guidances/UCM193282 (2009).
Wolffram, I. et al. Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios. J. Hepatol. 62, 1256–1264 (2015).
Mahajan, R., Liu, S. J., Klevens, R. M. & Holmberg, S. D. Indications for testing among reported cases of HCV infection from enhanced hepatitis surveillance sites in the United States, 2004–2010. Am. J. Public Health 103, 1445–1449 (2013).
Easterbrook, P. J. & WHO Guidelines Development Group. Who to test and how to test for chronic hepatitis C infection — 2016 WHO testing guidance for low- and middle-income countries. J. Hepatol. 65, S46–S66 (2016).
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. WHOwww.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (2016).
Baumert, T. F., Fauvelle, C., Chen, D. Y. & Lauer, G. M. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J. Hepatol. 61, S34–S44 (2014).
Ball, J. K., Tarr, A. W. & McKeating, J. A. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res. 105, 100–111 (2014).
Chapman, L. E. et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events — United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 57, 1–21 (2008).
Corey, K. E. et al. Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers. Infect. Control Hosp. Epidemiol. 30, 1000–1005 (2009).
Grebely, J. et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect. Dis. 12, 408–414 (2012).
Petta, S. & Craxi, A. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int. 35 (Suppl. 1), 4–10 (2015).
Deterding, K. et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect. Dis. 17, 215–222 (2017).
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C. EASL http://www.easl.eu/medias/cpg/HCV2016/Summary.pdf (2016). These are the HCV Clinical Practice Guidelines of the EASL, which are regularly updated online (www.easl.eu).
AASLD–IDSA. Recommendations for testing, managing, and treating hepatitis C. HCV Guidelines http://www.hcvguidelines.org (accessed 1 Dec 2016). These are the joint HCV Clinical Practice Guidelines by the AASLD and the IDSA, which are regularly updated online (www.hcvguidelines.org).
Jaeckel, E. et al. Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 345, 1452–1457 (2001). This paper highlights that early treatment of acute HCV infection can prevent chronicity.
Wiegand, J. et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 43, 250–256 (2006).
Deterding, K. et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect. Dis. 13, 497–506 (2013).
Deterding, K. et al. Six weeks of sofosbuvir/ledipasvir (SOF/LDV) are sufficient to treat acute hepatitis C virus genotype 1 monoinfection: the HepNet acute HCV IV study. J. Hepatol. 64, S211 (2016).
Rockstroh, J. K. et al. Ledipasvir/sofosbuvir for 6 weeks in HIV-infected patients with acute HCV infection. CROI Conferencehttp://www.croiconference.org/sessions/ledipasvirsofosbuvir-6-weeks-hiv-infected-patients-acute-hcv-infection (2016).
Zeuzem, S. et al. Grazoprevir–elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis c virus genotype 1, 4, or 6 infection: a randomized trial. Ann. Intern. Med. 163, 1–13 (2015).
Feld, J. J. et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 373, 2599–2607 (2015).
Foster, G. R. et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 373, 2608–2617 (2015).
Curry, M. P. et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 373, 2618–2628 (2015).
George Lau, M. et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: a phase 2, open-label, proof-of-concept study. Lancet Gastroenterol. Hepatol. 1, 97–104 (2016).
Kim, A. Y. & Chung, R. T. Coinfection with HIV-1 and HCV — a one–two punch. Gastroenterology 137, 795–814 (2009).
Qurishi, N. et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 362, 1708–1713 (2003).
Townsend, K. S. et al. High efficacy of sofosbuvir/ledipasvir for the treatment of HCV genotype 1 in patients coinfected with HIV on or off antiretroviral therapy: results from the NIAID ERADICATE trial. Hepatology 60, 240a–241a (2014).
Wyles, D. L. et al. TURQUOISE-I: 94% SVR12 in HCV/HIV-1 coinfected patients treated with ABT-450/r/ombitasvir, dasabuvir and ribavirin. Hepatology 60, 1136a–1137a (2014).
Wyles, D. et al. Sofosbuvir/velpatasvir fixed dose combination for 12 weeks in patients co-infected with HCV and HIV-1: the phase 3 ASTRAL-5 study. J. Hepatol. 64, S188 (2016).
Nakata, S. et al. Hepatitis-C and hepatitis-B virus-infections in populations at low or high-risk in Ho-Chi-Minh and Hanoi, Vietnam. J. Gastroenterol. Hepatol. 9, 416–419 (1994).
Conway, M. et al. Prevalence of antibodies to hepatitis-C in dialysis patients and transplant recipients with possible routes of transmission. Nephrol. Dial. Transplant. 7, 1226–1229 (1992).
Blackmore, T. K., Stace, N. H., Maddocks, P. & Hatfield, P. Prevalence of antibodies to hepatitis-C virus in patients receiving renal replacement therapy, and in the staff caring for them. Aust. N. Z. J. Med. 22, 353–357 (1992).
Chung, R. T. et al. Hepatitis C guidance: AASLD–IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62, 932–954 (2015).
Charlton, M. et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 148, 108–117 (2015).
Manns, M. et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect. Dis. 16, 685–697 (2016).
Hezode, C. et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology 147, 132–142.e4 (2014).
Flamm, S. L. et al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a prospective, multicenter study. Hepatology 60, 320a–321a (2014).
Charlton, M. et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 149, 649–659 (2015).
Pellicelli, A. M. et al. Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig. Liver Dis. 46, 923–927 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02097966 (2016).
Thuluvath, P. J. et al. Liver transplantation in the United States, 1999–2008. Am. J. Transplant. 10, 1003–1019 (2010).
Berenguer, M. et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J. Hepatol. 32, 673–684 (2000).
Terrault, N. Liver transplantation in the setting of chronic HCV. Best Pract. Res. Clin. Gastroenterol. 26, 531–548 (2012).
Berenguer, M. et al. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am. J. Transplant. 8, 679–687 (2008).
Terrault, N. A. & Berenguer, M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 12, 1192–1204 (2006).
Price, J. C. & Terrault, N. A. Treatment of hepatitis C in liver transplant patients: interferon out, direct antiviral combos in. Liver Transpl. 21, 423–434 (2015).
Fernández-Carrillo, E. A. Treatment of hepatitis C virus in patients with advanced cirrhosis: always justified? Analysis of the Hepa-C Registry. J. Hepatol. 64, S133 (2016).
Wiesner, R. H., Sorrell, M., Villamil, F. & International Liver Transplantation Society Expert Panel. Report of the first international Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 9, S1–S9 (2003).
Curry, M. P. et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 148, 100–107.e1 (2015).
Manns, M. et al. High efficacy of ledipasvir/sofosbuvir plus ribavirin among patients with decompensated cirrhosis who underwent liver transplant during participation in the SOLAR-1 and -2 studies. NATAPhttp://www.natap.org/2016/EASL/EASL_71.htm (2016).
Forns, X. et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C including fibrosing cholestatic hepatitis following liver transplantation. Hepatology 61, 1485–1494 (2014).
Spiegel, B. M. et al. The impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Gastroenterology 128, A749–A750 (2005).
Ware, J. E. & Kosinski, M. Interpreting SF-36 summary health measures: a response. Qual. Life Res. 10, 405–413 (2001).
Younossi, Z. M., Guyatt, G., Kiwi, M., Boparai, N. & King, D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 45, 295–300 (1999).
Gnanasakthy, A. et al. A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health 15, 437–442 (2012).
Younossi, Z. M. et al. The impact of hepatitis C burden: an evidence-based approach. Aliment. Pharmacol. Ther. 39, 518–531 (2014).
Afendy, A. et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 30, 469–476 (2009).
Kallman, J. et al. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig. Dis. Sci. 52, 2531–2539 (2007).
Gerber, L. et al. Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 14, 156–164.e3 (2016).
Younossi, Z. M. Chronic liver disease and health-related quality of life. Gastroenterology 120, 305–307 (2001).
Younossi, Z. M. et al. Sofosbuvir and velpatasvir combination improves outcomes reported by patients with HCV infection, without or with compensated or decompensated cirrhosis. Clin. Gastroenterol. Hepatol.http://dx.doi.org/10.1016/j.cgh.2016.10.037 (2016).
Younossi, Z. M. et al. Association of work productivity with clinical and patient-reported factors in patients infected with hepatitis C virus. J. Viral Hepat. 23, 623–630 (2016).
Younossi, I., Weinstein, A., Stepanova, M., Hunt, S. & Younossi, Z. M. Mental and emotional impairment in patients with hepatitis C is related to lower work productivity. Psychosomatics 57, 82–88 (2016).
Patel, K. & McHutchison, J. G. Initial treatment for chronic hepatitis C: current therapies and their optimal dosing and duration. Cleve. Clin. J. Med. 71, S8–S12 (2004).
Shehab, T. M. et al. Effectiveness of interferon alpha-2b and ribavirin combination therapy in the treatment of naive chronic hepatitis C patients in clinical practice. Clin. Gastroenterol. Hepatol. 2, 425–431 (2004).
Bruno, R. et al. OPERA: responses to peginterferon and ribavirin therapy in a subgroup of interferon-naive patients with HIV/HCV genotype 2/3 co-infection in Italy. Liver Int. 35, 120–129 (2015).
Younossi, Z. M., Singer, M. E., Mir, H. M., Henry, L. & Hunt, S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J. Hepatol. 60, 530–537 (2014).
Younossi, Z. M. et al. Sofosbuvir and ledipasvir improve patient-reported outcomes in patients co-infected with hepatitis C and human immunodeficiency virus. J. Viral Hepat. 23, 857–865 (2016).
John-Baptiste, A. A. et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am. J. Gastroenterol. 104, 2439–2448 (2009).
Reilly, M. C., Zbrozek, A. S. & Dukes, E. M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4, 353–365 (1993).
Escorpizo, R. et al. Worker productivity outcome measures in arthritis. J. Rheumatol. 34, 1372–1380 (2007).
Tang, K. et al. Worker productivity outcome measures: OMERACT filter evidence and agenda for future research. J. Rheumatol. 41, 165–176 (2014).
Younossi, Z. et al. Sustained virologic response with ledipasvir (LDV) and sofosbuvir (SOF) regimens leads to substantial improvement in patient-reported outcomes (PROs) among chronic hepatitis C (CHC) patients with early hepatic fibrosis as well as those with advanced hepatic fibrosis. Hepatology 60, 892a–893a (2014).
Baran, R. W., Xie, W. G., Liu, Y., Cohen, D. E. & Gooch, K. L. Health-related quality of life (HRQoL), health state, function and wellbeing of chronic HCV patients treated with interferon-free, oral DAA regimens: patient reported outcome (PRO) results from the AVIATOR study. Hepatology 58, 750a–751a (2013).
Scott, J. et al. Fatigue during treatment for hepatitis C virus: results of self-reported fatigue severity in two phase IIb studies of simeprevir treatment in patients with hepatitis C virus genotype 1 infection. BMC Infect. Dis. 14, 465 (2014).
Loria, A. et al. Multiple factors predict physical performance in people with chronic liver disease. Am. J. Phys. Med. Rehabil. 93, 470–476 (2014).
Younossi, Z. M. et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J. Hepatol. 61, 228–234 (2014).
Younossi, Z. M. et al. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from ASTRAL-1 placebo-controlled trial. J. Hepatol. 65, 33–39 (2016).
Younossi, Z. M. et al. The impact of hepatitis C virus outside the liver: evidence from Asia. Liver Int. 37, 159–172 (2017).
Bouliere, M. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks as a salvage regimen in NS5A inhibitor-experienced patients with genotype 1–6 infection: the phase 3 POLARIS-1 study. Hepatology 64, 102AA (2016).
Zeuzem, S. A. Randomized, controlled, phase 3 trial of sofosbuvir/velpatasvir/voxilaprevir or sofosbuvir/velpatasvir for 12 weeks in direct acting antiviral-experienced patients with genotype 1–6 HCV infection: the POLARIS-4 study. Hepatology 64, 59A (2016).
Freeman, J. et al. High sustained virological response rates using generic direct acting antiviral treatment for hepatitis c, imported into Australia. J. Hepatol. 64, S209 (2016).
Reig, M. et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 65, 719–726 (2016).
Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1, 1–85 (1964).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649 (1973).
Cornpropst, M. T. et al. The effect of renal impairment and end stage renal disease on the single-dose pharmacokinetics of Psi-7977. J. Hepatol. 56, S433 (2012).
Gane, E. J. et al. Safety, anti-viral efficacy and pharmacokinetics (PK) of sofosbuvir (SOF) in patients with severe renal impairment. Hepatology 60, 667a (2014).
Garimella, T. et al. The effect of renal impairment on single-dose pharmacokinetics of daclatasvir, an HCV NS5A inhibitor. J. Viral Hepatitis 21, 32 (2014).
Khatri, A. et al. The pharmacokinetics and safety of the direct acting antiviral regimen of ABT-450/r, ombitasvir with/without dasabuvir in subjects with mild, moderate and severe renal impairment compared to subjects with normal renal function. Hepatology 60, 320a (2014).
Roth, D. et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 386, 1537–1545 (2015).
Hoofnagle, J. H. Toward universal vaccination against hepatitis B virus. N. Engl. J. Med. 321, 1333–1334 (1989).
McHutchison, J. G. et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339, 1485–1492 (1998). This paper establishes IFN-α2b plus ribavirin as a new standard of care between 1998 and 2001.
Poynard, T. et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352, 1426–1432 (1998). This study establishes IFN-α2b plus ribavirin as standard of care for patients outside the United States.
Zeuzem, S. et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N. Engl. J. Med. 343, 1666–1672 (2000). This study provides proof of concept that PEG-IFN-α2a is superior to non-PEG-IFN.
Fried, M. W. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347, 975–982 (2002).
Manns, M. P. et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001). This study establishes PEG-IFN-α2b plus ribavirin as a new standard of care between 2001 and 2011.
Saraswat, V. et al. Historical epidemiology of hepatitis C virus (HCV) in select countries — volume 2. J. Viral Hepatitis 22, 6–25 (2015).
Attaullah, S., Khan, S. & Ali, I. Hepatitis C virus genotypes in Pakistan: a systemic review. Virol. J. 8, 433 (2011).
Abdel-Hamid, M. et al. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J. Gen. Virol. 88, 1526–1531 (2007).
El-Zayadi, A., Simmonds, P., Dabbous, H. & Selim, O. Hepatitis C virus genotypes among HCV-chronic liver disease patients in Egypt: a leading trial. J. Egypt Public Health Assoc. 69, 327–334 (1994).
Ray, S. C., Arthur, R. R., Carella, A., Bukh, J. & Thomas, D. L. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 182, 698–707 (2000).
Prabdial-Sing, N., Puren, A. J., Mahlangu, J., Barrow, P. & Bowyer, S. M. Hepatitis C virus genotypes in two different patient cohorts in Johannesburg, South Africa. Arch. Virol. 153, 2049–2058 (2008).
Smuts, H. E. & Kannemeyer, J. Genotyping of hepatitis C virus in South Africa. J. Clin. Microbiol. 33, 1679–1681 (1995).
Rao, H. et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 29, 545–553 (2014).
Akkarathamrongsin, S. et al. Seroprevalence and genotype of hepatitis C virus among immigrant workers from Cambodia and Myanmar in Thailand. Intervirology 54, 10–16 (2011).
Leung, N., Chu, C. & Tam, J. S. Viral hepatitis C in Hong Kong. Intervirology 49, 23–27 (2006).
Hubschen, J. M. et al. High genetic diversity including potential new subtypes of hepatitis C virus genotype 6 in Lao People's Democratic Republic. Clin. Microbiol. Infect. 17, E30–E34 (2011).
Lwin, A. A. et al. Hepatitis C virus genotype distribution in Myanmar: predominance of genotype 6 and existence of new genotype 6 subtype. Hepatol. Res. 37, 337–345 (2007).
Pham, V. H. et al. Very high prevalence of hepatitis C virus genotype 6 variants in southern Vietnam: large-scale survey based on sequence determination. Jpn J. Infect. Dis. 64, 537–539 (2011).
Acknowledgements
The authors thank S. Hardtke and P. Solbach, Hannover Medical School, Hannover, Germany, for editorial assistance and M. Cornberg, Hannover Medical School for helpful discussions.
Author information
Authors and Affiliations
Contributions
Introduction (M.P.M.); Epidemiology (H.R.); Mechanisms/pathophysiology (J.-M.P.); Diagnosis, screening and prevention (J.-M.P. and M.B.); Management (M.P.M., E.G. and N.T.); Quality of life (Z.Y.); Outlook (M.P.M.); Overview of Primer (M.P.M.).
Corresponding author
Ethics declarations
Competing interests
M.P.M. has received research grants and or served as an adviser for Roche, Bristol-Myers Squibb (BMS), Gilead, Boehringer Ingelheim, Novartis, Merck, Janssen, GlaxoSmithKline (GSK), Biotest and AbbVie. M.B. has served as a speaker and/or adviser of AbbVie, Gilead, Janssen, Merck and BMS. E.G. has served as an adviser for Roche, Gilead, Janssen, Novira, AbbVie, Novartis, Achillion, Merck and Alios. J.-M.P. has received research grants from Gilead Sciences and AbbVie. He has served as an adviser for AbbVie, BMS, Gilead, Janssen and Merck. H.R. has received research funds from Gilead and AbbVie. N.T. has received research grants and/or served as an adviser for Gilead, Cocrystal Pharma, BMS, AbbVie, Merck and Echosens North America Inc. She received royalty from UpToDate and is involved in continuing medical education and the development of educational material for CCO Hepatitis, Practice Point Communications and Focus Medical Communications. Z.Y. has received research funds from Gilead, BMS and AbbVie and is a consultant or an adviser to BMS, Gilead, GSK, Intercept and Tobira.
Rights and permissions
About this article
Cite this article
Manns, M., Buti, M., Gane, E. et al. Hepatitis C virus infection. Nat Rev Dis Primers 3, 17006 (2017). https://doi.org/10.1038/nrdp.2017.6
Published:
DOI: https://doi.org/10.1038/nrdp.2017.6
This article is cited by
-
Unraveling the dynamic mechanisms of natural killer cells in viral infections: insights and implications
Virology Journal (2024)
-
Viral hepatitis–induced acute liver failure
Indian Journal of Gastroenterology (2024)
-
Therapeutic potential of oleanolic acid in liver diseases
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
A time-series analysis of morbidity and mortality of viral hepatitis in Venezuela, 1990–2016
BMC Infectious Diseases (2023)
-
Redefining serological diagnostics with immunoaffinity proteomics
Clinical Proteomics (2023)