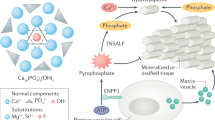
Abstract
Skeletal deformity and bone fragility are the hallmarks of the brittle bone dysplasia osteogenesis imperfecta. The diagnosis of osteogenesis imperfecta usually depends on family history and clinical presentation characterized by a fracture (or fractures) during the prenatal period, at birth or in early childhood; genetic tests can confirm diagnosis. Osteogenesis imperfecta is caused by dominant autosomal mutations in the type I collagen coding genes (COL1A1 and COL1A2) in about 85% of individuals, affecting collagen quantity or structure. In the past decade, (mostly) recessive, dominant and X-linked defects in a wide variety of genes encoding proteins involved in type I collagen synthesis, processing, secretion and post-translational modification, as well as in proteins that regulate the differentiation and activity of bone-forming cells have been shown to cause osteogenesis imperfecta. The large number of causative genes has complicated the classic classification of the disease, and although a new genetic classification system is widely used, it is still debated. Phenotypic manifestations in many organs, in addition to bone, are reported, such as abnormalities in the cardiovascular and pulmonary systems, skin fragility, muscle weakness, hearing loss and dentinogenesis imperfecta. Management involves surgical and medical treatment of skeletal abnormalities, and treatment of other complications. More innovative approaches based on gene and cell therapy, and signalling pathway alterations, are under investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marini, J. C. et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 28, 209–221 (2007). A paper that provides the best genotype–phenotype correlation for collagen mutations.
Marini, J. C. in Nelson Textbook of Pediatrics (eds Kliegman, R. M., Stanton, B., St. Geme, J., Schor, N. & Behrman, R. E. ) 2437–2440 (Elsevier Health Sciences, 2011).
Kang, H., Aryal, A. C. S. & Marini, J. C. Osteogenesis imperfecta: new genes reveal novel mechanisms in bone dysplasia. Transl Res. 181, 27–48 (2017).
Forlino, A. & Marini, J. C. Osteogenesis imperfecta. Lancet 387, 1657–1671 (2016). An up-to-date review of the genetics of osteogenesis imperfecta.
Orioli, I. M., Castilla, E. E. & Barbosa-Neto, J. G. The birth prevalence rates for the skeletal dysplasias. J. Med. Genet. 23, 328–332 (1986).
Stevenson, D. A., Carey, J. C., Byrne, J. L., Srisukhumbowornchai, S. & Feldkamp, M. L. Analysis of skeletal dysplasias in the Utah population. Am. J. Med. Genet. A 158A, 1046–1054 (2012).
Folkestad, L. et al. Mortality and causes of death in patients with osteogenesis imperfecta: a register-based nationwide cohort study. J. Bone Miner. Res. 31, 2159–2166 (2016).
Kuurila, K., Kaitila, I., Johansson, R. & Grénman, R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. Ann. Otol. Rhinol. Laryngol. 111, 939–946 (2002).
Bardai, G., Moffatt, P., Glorieux, F. H. & Rauch, F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta: diagnostic yield and mutation spectrum. Osteoporos Int. 27, 3607–3613 (2016).
Ward, L. et al. Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone 31, 12–18 (2002).
Cabral, W. A. et al. A founder mutation in LEPRE1 carried by 1.5% of West Africans and 0.4% of African Americans causes lethal recessive osteogenesis imperfecta. Genet. Med. 14, 543–551 (2012).
Kurt-Sukur, E. D., Simsek-Kiper, P. O., Utine, G. E., Boduroglu, K. & Alanay, Y. Experience of a skeletal dysplasia registry in Turkey: a five-years retrospective analysis. Am. J. Med. Genet. A 167A, 2065–2074 (2015).
Morello, R. et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127, 291–304 (2006).
Volodarsky, M. et al. A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum. Mutat. 34, 582–586 (2013).
Alanay, Y. et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am. J. Hum. Genet. 86, 551–559 (2010).
Pyott, S. M. et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am. J. Hum. Genet. 92, 590–597 (2013).
Ishikawa, Y. & Bachinger, H. P. A molecular ensemble in the rER for procollagen maturation. Biochim. Biophys. Acta 1833, 2479–2491 (2013). An exhaustive summary of collagen biochemistry.
Myllyharju, J. & Kivirikko, K. I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20, 33–43 (2004).
Vranka, J. A., Sakai, L. Y. & Bachinger, H. P. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J. Biol. Chem. 279, 23615–23621 (2004).
Weis, M. A. et al. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J. Biol. Chem. 285, 2580–2590 (2010).
Forlino, A., Cabral, W. A., Barnes, A. M. & Marini, J. C. New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 7, 540–557 (2011).
Hudson, D. M., Kim, L. S., Weis, M., Cohn, D. H. & Eyre, D. R. Peptidyl 3-hydroxyproline binding properties of type I collagen suggest a function in fibril supramolecular assembly. Biochemistry 51, 2417–2424 (2012). A careful hypothesis of the role of prolyl 3-hydroxylation.
Ishikawa, Y., Wirz, J., Vranka, J. A., Nagata, K. & Bachinger, H. P. Biochemical characterization of the prolyl 3-hydroxylase 1·cartilage-associated protein·cyclophilin B complex. J. Biol. Chem. 284, 17641–17647 (2009).
Zeng, B. et al. Chicken FK506-binding protein, FKBP65 a member of the FKBP family of peptidylprolyl cis–trans isomerases, is only partially inhibited by FK506. Biochem. J. 330, 109–114 (1998).
Saga, S., Nagata, K., Chen, W. T. & Yamada, K. M. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J. Cell Biol. 105, 517–527 (1987).
Satoh, M., Hirayoshi, K., Yokota, S., Hosokawa, N. & Nagata, K. Intracellular interaction of collagen-specific stress protein HSP47 with newly synthesized procollagen. J. Cell Biol. 133, 469–483 (1996).
Thomson, C. A. & Ananthanarayanan, V. S. Structure–function studies on hsp47: pH-dependent inhibition of collagen fibril formation in vitro. Biochem. J. 349, 877–883 (2000).
Sillence, D. O., Rimoin, D. L. & Danks, D. M. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig. Art. Ser. 15, 113–129 (1979).
Ishida, Y. et al. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol. Biol. Cell 20, 2744–2754 (2009).
Forlino, A. et al. Differential expression of both extracellular and intracellular proteins is involved in the lethal or nonlethal phenotypic variation of BrtlIV, a murine model for osteogenesis imperfecta. Proteomics 7, 1877–1891 (2007).
Bianchi, L. et al. Differential response to intracellular stress in the skin from osteogenesis imperfecta Brtl mice with lethal and non lethal phenotype: a proteomic approach. J. Proteomics 75, 4717–4733 (2012).
Gioia, R. et al. Impaired osteoblastogenesis in a murine model of dominant osteogenesis imperfecta: a new target for osteogenesis imperfecta pharmacological therapy. Stem Cells 30, 1465–1476 (2012). A paper that delineates defective osteoblast development as part of the pathophysiology of osteogenesis imperfecta.
Chu, M. & Prockop, D. J. in Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects 2nd edn (eds Royce, P. M. & Steinmann, B. ) 223–248 (Wiley-Liss, 2002).
Symoens, S. et al. Type I procollagen C-propeptide defects: study of genotype–phenotype correlation and predictive role of crystal structure. Hum. Mutat. 35, 1330–1341 (2014).
Cabral, W. A. et al. Type I collagen triplet duplication mutation in lethal osteogenesis imperfecta shifts register of alpha chains throughout the helix and disrupts incorporation of mutant helices into fibrils and extracellular matrix. J. Biol. Chem. 278, 10006–10012 (2003).
Sweeney, S. M. et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 283, 21187–21197 (2008).
Orgel, J. P., San Antonio, J. D. & Antipova, O. Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res. 52, 2–17 (2011).
Canty, E. G. & Kadler, K. E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 118, 1341–1353 (2005).
Cabral, W. A. et al. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers–Danlos syndrome by interference with N-propeptide processing. J. Biol. Chem. 280, 19259–19269 (2005). A paper that delineates the mechanism of osteogenesis imperfecta resulting from defects in the cleavage of the collagen N-propeptide.
Malfait, F. et al. Helical mutations in type I collagen that affect the processing of the amino-propeptide result in an osteogenesis imperfecta/Ehlers–Danlos syndrome overlap syndrome. Orphanet J. Rare Dis. 8, 78 (2013).
Wiestner, M. et al. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J. Biol. Chem. 254, 7016–7023 (1979).
Oganesian, A. et al. The NH2-terminal propeptide of type I procollagen acts intracellularly to modulate cell function. J. Biol. Chem. 281, 38507–38518 (2006).
Byers, P. H. et al. Ehlers–Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen. Am. J. Med. Genet. 72, 94–105 (1997).
Pace, J. M. et al. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. J. Biol. Chem. 283, 16061–16067 (2008).
Cundy, T., King, A. & Byers, P. H. A novel disorder of type I collagen characterized by high bone mass, a mineralization defect and tendon calcification. Calcif. Tissue Int. 82, S41 (2008).
Martinez-Glez, V. et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum. Mutat. 33, 343–350 (2012).
Asharani, P. V. et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 90, 661–674 (2012).
Kessler, E., Takahara, K., Biniaminov, L., Brusel, M. & Greenspan, D. S. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271, 360–362 (1996).
Li, S. W. et al. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc. Natl Acad. Sci. USA 93, 5127–5130 (1996).
Pappano, W. N., Steiglitz, B. M., Scott, I. C., Keene, D. R. & Greenspan, D. S. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol. Cell. Biol. 23, 4428–4438 (2003).
Imamura, Y., Steiglitz, B. M. & Greenspan, D. S. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J. Biol. Chem. 273, 27511–27517 (1998).
Uzel, M. I. et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J. Biol. Chem. 276, 22537–22543 (2001).
Syx, D. et al. Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J. Bone Miner. Res. 30, 1445–1456 (2015).
Scott, I. C. et al. Bone morphogenetic protein-1 processes probiglycan. J. Biol. Chem. 275, 30504–30511 (2000).
Vadon- Le Goff, S., Hulmes, D. J. & Moali, C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol. 44–46, 14–23 (2015).
Barnes, A. M. et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N. Engl. J. Med. 355, 2757–2764 (2006).
Cabral, W. A. et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 39, 359–365 (2007).
van Dijk, F. S. et al. PPIB mutations cause severe osteogenesis imperfecta. Am. J. Hum. Genet. 85, 521–527 (2009).
Chang, W., Barnes, A. M., Cabral, W. A., Bodurtha, J. N. & Marini, J. C. Prolyl 3-hydroxylase 1 and CRTAP are mutually stabilizing in the endoplasmic reticulum collagen prolyl 3-hydroxylation complex. Hum. Mol. Genet. 19, 223–234 (2010). A study that delineates the mutual protection relationship of P3H1–CRTAP in the prolyl-3-hydroxylation complex.
van Dijk, F. S. et al. Lethal/severe osteogenesis imperfecta in a large family: a novel homozygous LEPRE1 mutation and bone histological findings. Pediatr. Dev. Pathol. 14, 228–234 (2011).
Caparros-Martin, J. A. et al. Clinical and molecular analysis in families with autosomal recessive osteogenesis imperfecta identifies mutations in five genes and suggests genotype–phenotype correlations. Am. J. Med. Genet. A 161A, 1354–1369 (2013).
Stephen, J. et al. Mutations in patients with osteogenesis imperfecta from consanguineous Indian families. Eur. J. Med. Genet. 58, 21–27 (2015).
Pyott, S. M. et al. Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Hum. Mol. Genet. 20, 1595–1609 (2011).
Barnes, A. M. et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N. Engl. J. Med. 362, 521–528 (2010).
Ishikawa, Y., Vranka, J., Wirz, J., Nagata, K. & Bachinger, H. P. The rough endoplasmic reticulum-resident FK506-binding protein FKBP65 is a molecular chaperone that interacts with collagens. J. Biol. Chem. 283, 31584–31590 (2008).
Nagata, K. HSP47 as a collagen-specific molecular chaperone: function and expression in normal mouse development. Semin. Cell Dev. Biol. 14, 275–282 (2003).
Christiansen, H. E. et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am. J. Hum. Genet. 86, 389–398 (2010).
Lindert, U. et al. Molecular consequences of the SERPINH1/HSP47 mutation in the Dachshund natural model of osteogenesis imperfecta. J. Biol. Chem. 290, 17679–17689 (2015).
Nagai, N. et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 150, 1499–1506 (2000).
Ishida, Y. et al. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol. Biol. Cell 17, 2346–2355 (2006).
Schwarze, U. et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum. Mol. Genet. 22, 1–17 (2013).
Kelley, B. P. et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J. Bone Miner. Res. 26, 666–672 (2011).
Setijowati, E. D. et al. A novel homozygous 5 bp deletion in FKBP10 causes clinically Bruck syndrome in an Indonesian patient. Eur. J. Med. Genet. 55, 17–21 (2012).
Zhou, P. et al. Novel mutations in FKBP10 and PLOD2 cause rare Bruck syndrome in Chinese patients. PLoS ONE 9, e107594 (2014).
Steinlein, O. K., Aichinger, E., Trucks, H. & Sander, T. Mutations in FKBP10 can cause a severe form of isolated osteogenesis imperfecta. BMC Med. Genet. 12, 152 (2011).
Barnes, A. M. et al. Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum. Mutat. 34, 1279–1288 (2013).
Venturi, G. et al. A novel splicing mutation in FKBP10 causing osteogenesis imperfecta with a possible mineralization defect. Bone 50, 343–349 (2012).
Moravej, H. et al. Bruck syndrome — a rare syndrome of bone fragility and joint contracture and novel homozygous FKBP10 mutation. Endokrynol. Pol. 66, 170–174 (2015).
Kivirikko, K. I. & Pihlajaniemi, T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325–398 (1998).
Puig-Hervas, M. T. et al. Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome — osteogenesis imperfecta phenotypic spectrum. Hum. Mutat. 33, 1444–1449 (2012).
Ha-Vinh, R. et al. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am. J. Med. Genet. A 131A, 115–120 (2004).
van der Slot, A. J. et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J. Biol. Chem. 278, 40967–40972 (2003).
Becker, J. et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am. J. Hum. Genet. 88, 362–371 (2011).
Homan, E. P. et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J. Bone Miner. Res. 26, 2798–2803 (2011).
Akiyama, T. et al. PEDF regulates osteoclasts via osteoprotegerin and RANKL. Biochem. Biophys. Res. Commun. 391, 789–794 (2010).
Semler, O. et al. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am. J. Hum. Genet. 91, 349–357 (2012).
Cho, T. J. et al. A single recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am. J. Hum. Genet. 91, 343–348 (2012).
Farber, C. R. et al. A novel IFITM5 mutation in severe atypical osteogenesis imperfecta type VI impairs osteoblast production of pigment epithelium-derived factor. J. Bone Miner. Res. 29, 1402–14011 (2014).
Hoyer-Kuhn, H. et al. A nonclassical IFITM5 mutation located in the coding region causes severe osteogenesis imperfecta with prenatal onset. J. Bone Miner. Res. 29, 1387–1391 (2014).
Glorieux, F. H. et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J. Bone Miner. Res. 15, 1650–1658 (2000).
Glorieux, F. H. et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J. Bone Miner. Res. 17, 30–38 (2002).
Lapunzina, P. et al. Identification of a frameshift mutation in osterix in a patient with recessive osteogenesis imperfecta. Am. J. Hum. Genet. 87, 110–114 (2010).
Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002).
Cabral, W. A. et al. Absence of the ER cation channel TMEM38B/TRIC-B disrupts intracellular calcium homeostasis and dysregulates collagen synthesis in recessive osteogenesis imperfecta. PLoS Genet. 12, e1006156 (2016).
Yamazaki, D. et al. Essential role of the TRIC-B channel in Ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development 136, 2355–2361 (2009).
Yazawa, M. et al. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 448, 78–82 (2007).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Keupp, K. et al. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 92, 565–574 (2013).
Laine, C. M. et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 368, 1809–1816 (2013).
Palomo, T. et al. Skeletal characteristics associated with homozygous and heterozygous WNT1 mutations. Bone 67, 63–70 (2014).
Willert, K. & Nusse, R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 4, a007864 (2012).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
Gong, Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001).
Ai, M., Heeger, S., Bartels, C. F. & Schelling, D. K. Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am. J. Hum. Genet. 77, 741–753 (2005).
Lara-Castillo, N. & Johnson, M. L. LRP receptor family member associated bone disease. Rev. Endocr. Metab. Disord. 16, 141–148 (2015).
Scopelliti, D., Orsini, R., Ventucci, E. & Carratelli, D. Van Buchem disease. Maxillofacial changes, diagnostic classification and general principles of treatment [Italian]. Minerva Stomatol. 48, 227–234 (1999).
Thomas, K. R., Musci, T. S., Neumann, P. E. & Capecchi, M. R. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell 67, 969–976 (1991).
Lindert, U. et al. MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta. Nat. Commun. 7, 11920 (2016).
Symoens, S. et al. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J.Rare Dis. 8, 154 (2013).
Murakami, T. et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 11, 1205–1211 (2009).
Rauch, F., Travers, R., Parfitt, A. M. & Glorieux, F. H. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 26, 581–589 (2000). A study that shows that the bone histomorphometry of patients with osteogenesis imperfecta caused by collagen defects shows high turnover.
Fratzl-Zelman, N. et al. Non-lethal type VIII osteogenesis imperfecta has elevated bone matrix mineralization. J. Clin. Endocrinol. Metab. 101, 3516–3525 (2016).
Boyde, A., Travers, R., Glorieux, F. H. & Jones, S. J. The mineralization density of iliac crest bone from children with osteogenesis imperfecta. Calcif. Tissue Int. 64, 185–190 (1999).
Roschger, P. et al. Evidence that abnormal high bone mineralization in growing children with osteogenesis imperfecta is not associated with specific collagen mutations. Calcif. Tissue Int. 82, 263–270 (2008). An article that shows that bone hypermineralization is a common feature of several types of osteogenesis imperfecta.
Weber, M. et al. Pamidronate does not adversely affect bone intrinsic material properties in children with osteogenesis imperfecta. Bone 39, 616–622 (2006).
Fratzl-Zelman, N. et al. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone 46, 820–826 (2010).
Fratzl-Zelman, N. et al. Unique micro- and nano-scale mineralization pattern of human osteogenesis imperfecta type VI bone. Bone 73, 233–241 (2015).
Misof, B. M. et al. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone 36, 150–158 (2005).
Vanleene, M. et al. Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone 50, 1317–1323 (2012).
Fratzl-Zelman, N. et al. Mineral particle size in children with osteogenesis imperfecta type I is not increased independently of specific collagen mutations. Bone 60, 122–128 (2014).
Paschalis, E. P. et al. Evidence for a role for nanoporosity and pyridinoline content in human mild osteogenesis imperfecta. J. Bone Miner. Res. 31, 1050–1059 (2016).
Hasegawa, K. et al. Impaired pyridinoline cross-link formation in patients with osteogenesis imperfecta. J. Bone Miner. Metab. 26, 394–399 (2008).
Eyre, D. R. & Weis, M. A. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif. Tissue Int. 93, 338–347 (2013).
Carriero, A. et al. How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone. J. Bone Miner. Res. 29, 1392–1401 (2014).
Kozloff, K. M. et al. Brittle IV mouse model for osteogenesis imperfecta IV demonstrates postpubertal adaptations to improve whole bone strength. J. Bone Miner. Res. 19, 614–622 (2004).
Wagermaier, W., Klaushofer, K. & Fratzl, P. Fragility of bone material controlled by internal interfaces. Calcif. Tissue Int. 97, 201–212 (2015).
Fratzl, P., Paris, O., Klaushofer, K. & Landis, W. J. Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle X-ray scattering. J. Clin. Invest. 97, 396–402 (1996).
Glorieux, F. H. et al. Normative data for iliac bone histomorphometry in growing children. Bone 26, 103–109 (2000).
Camacho, N. P. et al. The material basis for reduced mechanical properties in oim mice bones. J. Bone Miner. Res. 14, 264–272 (1999).
Misof, K., Landis, W. J., Klaushofer, K. & Fratzl, P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J. Clin. Invest. 100, 40–45 (1997).
Andriotis, O. G. et al. Structure–mechanics relationships of collagen fibrils in the osteogenesis imperfecta mouse model. J. R. Soc. Interface 12, 20150701 (2015).
Rodriguez-Florez, N. et al. An investigation of the mineral in ductile and brittle cortical mouse bone. J. Bone Miner. Res. 30, 786–795 (2015).
Bishop, N. Bone material properties in osteogenesis imperfecta. J. Bone Miner. Res. 31, 699–708 (2016).
Sillence, D., Senn, A. & Danks, D. Genetic heterogeneity in osteogenesis imperfecta. J. Med. Genet. 16, 101–116 (1979).
Bonafe, L. et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A 167A, 2869–2892 (2015).
Marlowe, A., Pepin, M. & Byers, P. Testing for osteogenesis imperfecta in cases of suspected non-accidental injury. J. Med. Genet. 39, 382–386 (2002).
van Dijk, F. S. et al. EMQN best practice guidelines for the laboratory diagnosis of osteogenesis imperfecta. Eur. J. Hum. Genet. 20, 11–19 (2012).
Marini, J. C. & Blissett, A. R. New genes in bone development: what's new in osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 98, 3095–3103 (2013).
Monti, E. et al. Current and emerging treatments for the management of osteogenesis imperfecta. Ther. Clin. Risk Manag. 6, 367–381 (2010).
Rauch, F. & Glorieux, F. H. Osteogenesis imperfecta. Lancet 363, 1377–1385 (2004).
Brizola, E., Staub, A. L. & Felix, T. M. Muscle strength, joint range of motion, and gait in children and adolescents with osteogenesis imperfecta. Pediatr. Phys. Ther. 26, 245–252 (2014).
Caudill, A. et al. Ankle strength and functional limitations in children and adolescents with type I osteogenesis imperfecta. Pediatr. Phys. Ther. 22, 288–295 (2010).
Sousa, T., Bompadre, V. & White, K. K. Musculoskeletal functional outcomes in children with osteogenesis imperfecta: associations with disease severity and pamidronate therapy. J. Pediatr. Orthop. 34, 118–122 (2014).
Takken, T. et al. Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. J. Pediatr. 145, 813–818 (2004).
Amako, M. et al. Functional analysis of upper limb deformities in osteogenesis imperfecta. J. Pediatr. Orthop. 24, 689–694 (2004).
Montpetit, K., Palomo, T., Glorieux, F. H., Fassier, F. & Rauch, F. Multidisciplinary treatment of severe osteogenesis imperfecta: functional outcomes at skeletal maturity. Arch. Phys. Med. Rehabil. 96, 1834–1839 (2015).
Montpetit, K. et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics 111, e601–e603 (2003).
Van Brussel, M. et al. Physical training in children with osteogenesis imperfecta. J. Pediatr. 152, 111–116.e1 (2008).
Biggin, A. & Munns, C. F. Osteogenesis imperfecta: diagnosis and treatment. Curr. Osteoporos Rep. 12, 279–288 (2014).
Land, C., Rauch, F., Montpetit, K., Ruck-Gibis, J. & Glorieux, F. H. Effect of intravenous pamidronate therapy on functional abilities and level of ambulation in children with osteogenesis imperfecta. J. Pediatr. 148, 456–460 (2006).
Harrington, J., Sochett, E. & Howard, A. Update on the evaluation and treatment of osteogenesis imperfecta. Pediatr. Clin. North Am. 61, 1243–1257 (2014).
Cintas, H. L. & Gerber, L. H. Children with Osteogenesis Imperfecta: Strategies to Enhance Performance (The Osteogenesis Imperfecta Foundation, 2005).
Hoyer-Kuhn, H. et al. A specialized rehabilitation approach improves mobility in children with osteogenesis imperfecta. J. Musculoskelet. Neuronal Interact. 14, 445–453 (2014).
Semler, O. et al. Results of a prospective pilot trial on mobility after whole body vibration in children and adolescents with osteogenesis imperfecta. Clin. Rehabil. 22, 387–394 (2008).
Esposito, P. W. in Osteogenesis Imperfecta in Operative Techniques in Pediatric Orthopaedics (eds Flynn J. M. & Wiesel, S. W. ) 259–269 (Philadelphia Lippincott Williams and Wilkins, 2011).
Enright, W. J. & Noonan, K. J. Bone plating in patients with type III osteogenesis imperfecta: results and complications. Iowa Orthop. J. 26, 37–40 (2006).
Joseph, B., Rebello, G. & Chandra Kant, B. The choice of intramedullary devices for the femur and the tibia in osteogenesis imperfecta. J. Pediatr. Orthop. B 14, 311–319 (2005).
Li, W. C., Kao, H. K., Yang, W. E., Chang, C. J. & Chang, C. H. Femoral non-elongating rodding in osteogenesis imperfecta — the importance of purchasing epiphyseal plate. Biomed. J. 38, 143–147 (2015).
Popkov, D. A., Kononovich, N. A., Mingazov, E. R., Shutov, R. B. & Barbier, D. Intramedullary elastic transphyseal tibial osteosynthesis and its effect on segmental growth [Russian]. Vestn. Ross. Akad. Med. Nauk 4, 441–449 (2015).
Ashby, E., Montpetit, K., Hamdy, R. C. & Fassier, F. Functional outcome of humeral rodding in children with osteogenesis imperfecta. J. Pediatr. Orthop.http://dx.doi.org/10.1097/BPO.0000000000000729 (2016).
Ashby, E., Montpetit, K., Hamdy, R. C. & Fassier, F. Functional outcome of forearm rodding in children with osteogenesis imperfecta. J. Pediatr. Orthop.http://dx.doi.org/10.1097/BPO.0000000000000724 (2016).
Ruck, J., Dahan-Oliel, N., Montpetit, K., Rauch, F. & Fassier, F. Fassier–Duval femoral rodding in children with osteogenesis imperfecta receiving bisphosphonates: functional outcomes at one year. J. Child. Orthop. 5, 217–224 (2011).
Sato, A., Ouellet, J., Muneta, T., Glorieux, F. H. & Rauch, F. Scoliosis in osteogenesis imperfecta caused by COL1A1/COL1A2 mutations — genotype–phenotype correlations and effect of bisphosphonate treatment. Bone 86, 53–57 (2016).
Rauch, F., Munns, C., Land, C. & Glorieux, F. H. Pamidronate in children and adolescents with osteogenesis imperfecta: effect of treatment discontinuation. J. Clin. Endocrinol. Metab. 91, 1268–1274 (2006).
Bishop, N. et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1424–1432 (2013).
Bishop, N. et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J. Bone Miner. Res. 25, 32–40 (2010).
Dwan, K., Phillipi, C. A., Steiner, R. D. & Basel, D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev. 7, CD005088 (2014).
Sakkers, R. et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet 363, 1427–1431 (2004).
Ward, L. M. et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J. Clin. Endocrinol. Metab. 96, 355–364 (2011).
Orwoll, E. S. et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J. Clin. Invest. 124, 491–498 (2014).
Hald, J. D., Evangelou, E., Langdahl, B. L. & Ralston, S. H. Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J. Bone Miner. Res. 30, 929–933 (2015).
Rijks, E. B. et al. Efficacy and safety of bisphosphonate therapy in children with osteogenesis imperfecta: a systematic review. Horm. Res. Paediatr. 84, 26–42 (2015). A set of three meta-analyses (references 167, 171 and 172) of bisphosphonate treatment trials that show equivocal effect on fractures in osteogenesis imperfecta.
Uveges, T. E. et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J. Bone Miner. Res. 24, 849–859 (2009).
Rauch, F., Travers, R., Plotkin, H. & Glorieux, F. H. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J. Clin. Invest. 110, 1293–1299 (2002).
Letocha, A. D. et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J. Bone Miner. Res. 20, 977–986 (2005).
Martin, E. & Shapiro, J. R. Osteogenesis imperfecta: epidemiology and pathophysiology. Curr. Osteoporos Rep. 5, 91–97 (2007).
McAllion, S. J. & Paterson, C. R. Causes of death in osteogenesis imperfecta. J. Clin. Pathol. 49, 627–630 (1996).
Widmann, R. F. et al. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine (Phila Pa 1976) 24, 1673–1678 (1999).
Falvo, K. A., Klain, D. B., Krauss, A. N., Root, L. & Auld, P. A. Pulmonary function studies in osteogenesis imperfecta. Am. Rev. Respir. Dis. 108, 1258–1260 (1973).
Thiele, F. et al. Cardiopulmonary dysfunction in the osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum. Mol. Genet. 21, 3535–3545 (2012).
de Vlaming, A. et al. Atrioventricular valve development: new perspectives on an old theme. Differentiation 84, 103–116 (2012).
McNeeley, M. F., Dontchos, B. N., Laflamme, M. A., Hubka, M. & Sadro, C. T. Aortic dissection in osteogenesis imperfecta: case report and review of the literature. Emerg. Radiol. 19, 553–556 (2012).
Bonilla Jimenez, V. et al. Cardiac abnormalities in osteogenesis imperfecta. Case–control echocardiographic study [Spanish]. Med. Clin. (Barc.) 135, 681–684 (2010).
Najib, M. Q. et al. Valvular heart disease in patients with osteogenesis imperfecta. J. Card. Surg. 28, 139–143 (2013).
Radunovic, Z., Wekre, L. L., Diep, L. M. & Steine, K. Cardiovascular abnormalities in adults with osteogenesis imperfecta. Am. Heart J. 161, 523–529 (2011).
Migliaccio, S. et al. Impairment of diastolic function in adult patients affected by osteogenesis imperfecta clinically asymptomatic for cardiac disease: casuality or causality? Int. J. Cardiol. 131, 200–203 (2009).
Jackson, S. C., Odiaman, L., Card, R. T., van der Bom, J. G. & Poon, M. C. Suspected collagen disorders in the bleeding disorder clinic: a case–control study. Haemophilia 19, 246–250 (2013).
Sasaki-Adams, D. et al. Neurosurgical implications of osteogenesis imperfecta in children. Report of 4 cases. J. Neurosurg. Pediatr. 1, 229–236 (2008).
Byra, P., Chillag, S. & Petit, S. Osteogenesis imperfecta and aortic dissection. Am. J. Med. Sci. 336, 70–72 (2008).
Swinnen, F. K. et al. Osteogenesis imperfecta: the audiological phenotype lacks correlation with the genotype. Orphanet J.Rare Dis. 6, 88 (2011).
Hartikka, H. et al. Lack of correlation between the type of COL1A1 or COL1A2 mutation and hearing loss in osteogenesis imperfecta patients. Hum. Mutat. 24, 147–154 (2004).
Swinnen, F. K., De Leenheer, E. M., Coucke, P. J., Cremers, C. W. & Dhooge, I. J. Audiometric, surgical, and genetic findings in 15 ears of patients with osteogenesis imperfecta. Laryngoscope 119, 1171–1179 (2009).
Swinnen, F. K. et al. Audiologic phenotype of osteogenesis imperfecta: use in clinical differentiation. Otol. Neurotol. 33, 115–122 (2012).
Takagi, Y. & Sasaki, S. A probable common disturbance in the early stage of odontoblast differentiation in dentinogenesis imperfecta type I and type II. J. Oral Pathol. 17, 208–212 (1988).
American Academy of Pediatric Dentristy. Guideline on dental management of heritable dental developmental anomalies. Pediatr. Dent. 35, E179–E184 (2013).
Camfield, P. & Camfield, C. Transition to adult care for children with chronic neurological disorders. Ann. Neurol. 69, 437–444 (2011).
Reid, G. J. et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics 113, e197–e205 (2004).
Orlando, L. A. et al. Implementing family health history risk stratification in primary care: impact of guideline criteria on populations and resource demand. Am. J. Med Genet. C Semin. Med. Genet. 166C, 24–33 (2014).
Roberts, T. T., Cepela, D. J., Uhl, R. L. & Lozman, J. Orthopaedic considerations for the adult with osteogenesis imperfecta. J. Am. Acad. Orthop. Surg. 24, 298–308 (2016).
Bishop, N. J. & Walsh, J. S. Osteogenesis imperfecta in adults. J. Clin. Invest. 124, 476–477 (2014).
Edouard, T., Glorieux, F. H. & Rauch, F. Predictors and correlates of vitamin D status in children and adolescents with osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 96, 3193–3198 (2011).
Balkefors, V., Mattsson, E., Pernow, Y. & Saaf, M. Functioning and quality of life in adults with mild-to-moderate osteogenesis imperfecta. Physiother. Res. Int. 18, 203–211 (2013).
Lindahl, K., Langdahl, B., Ljunggren, O. & Kindmark, A. Treatment of osteogenesis imperfecta in adults. Eur. J. Endocrinol. 171, R79–R90 (2014).
Saeves, R. et al. Oral findings in adults with osteogenesis imperfecta. Spec. Care Dentist 29, 102–108 (2009).
Mauri, L. et al. Expanding the clinical spectrum of COL1A1 mutations in different forms of glaucoma. Orphanet J.Rare Dis. 11, 108 (2016).
Batzdorf, U. Clinical presentation and alternative diagnoses in the adult population. Neurosurg. Clin. N. Am. 26, 515–517 (2015).
Venugopala, D., Babu, S., Korath, M. P. & Jagadeesan, K. Renal stone disease as extra skeletal manifestation of osteogenesis imperfecta. J. Assoc. Physicians India 48, 1027–1028 (2000).
Vetter, U. et al. Osteogenesis imperfecta in childhood: cardiac and renal manifestations. Eur. J. Pediatr. 149, 184–187 (1989).
The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc. Sci. Med. 41, 1403–1409 (1995).
Widmann, R. F., Laplaza, F. J., Bitan, F. D., Brooks, C. E. & Root, L. Quality of life in osteogenesis imperfecta. Int. Orthop. 26, 3–6 (2002).
Dahan-Oliel, N. et al. Quality of life in osteogenesis imperfecta: a mixed-methods systematic review. Am. J. Med. Genet. A 170A, 62–76 (2016).
Dogba, M. J. et al. The impact of severe osteogenesis imperfecta on the lives of young patients and their parents — a qualitative analysis. BMC Pediatr. 13, 153 (2013).
Fano, V., del Pino, M., Rodriguez Celin, M., Buceta, S. & Obregon, M. G. Osteogenesis imperfecta: quality of life in children [Spanish]. Arch. Argent. Pediatr. 111, 328–331 (2013).
Kok, D. H. et al. Quality of life in children with osteogenesis imperfecta treated with oral bisphosphonates (olpadronate): a 2-year randomized placebo-controlled trial. Eur. J. Pediatr. 166, 1155–1161 (2007).
Lowing, K., Astrom, E., Oscarsson, K. A., Soderhall, S. & Eliasson, A. C. Effect of intravenous pamidronate therapy on everyday activities in children with osteogenesis imperfecta. Acta Paediatr. 96, 1180–1183 (2007).
Seikaly, M. G. et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J. Pediatr. Orthop. 25, 786–791 (2005).
Vanz, A. P., Felix, T. M., da Rocha, N. S. & Schwartz, I. V. Quality of life in caregivers of children and adolescents with osteogenesis imperfecta. Health Qual. Life Outcomes 13, 41 (2015).
Szczepaniak-Kubat, A., Kurnatowska, O., Jakubowska-Pietkiewicz, E. & Chlebna-Sokol, D. Assessment of quality of life of parents of children with osteogenesis imperfecta. Adv. Clin. Exp. Med. 21, 99–104 (2012).
Hill, C. L., Baird, W. O. & Walters, S. J. Quality of life in children and adolescents with osteogenesis imperfecta: a qualitative interview based study. Health Qual. Life Outcomes 12, 54 (2014).
Rauch, F., Lalic, L., Glorieux, F. H., Moffatt, P. & Roughley, P. Targeted sequencing of a pediatric metabolic bone gene panel using a desktop semiconductor next-generation sequencer. Calcif. Tissue Int. 95, 323–331 (2014).
Cole, D. E. Psychosocial aspects of osteogenesis imperfecta: an update. Am. J. Med. Genet. 45, 207–211 (1993).
Ashournia, H., Johansen, F. T., Folkestad, L., Diederichsen, A. C. & Brixen, K. Heart disease in patients with osteogenesis imperfecta — a systematic review. Int. J. Cardiol. 196, 149–157 (2015).
Radunovic, Z. & Steine, K. Prevalence of cardiovascular disease and cardiac symptoms: left and right ventricular function in adults with osteogenesis imperfecta. Can. J. Cardiol. 31, 1386–1392 (2015).
van der Kley, F., Delgado, V., Ajmone Marsan, N. & Schalij, M. J. Transcatheter mitral valve repair in osteogenesis imperfecta associated mitral valve regurgitation. Heart Lung Circ. 23, e169–e171 (2014).
Gebken, J. et al. Increased cell surface expression of receptors for transforming growth factor-beta on osteoblasts from patients with osteogenesis imperfecta. Pathobiology 68, 106–112 (2000).
Grafe, I. et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 20, 670–675 (2014).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
Sinder, B. P. et al. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J. Bone Miner. Res. 28, 73–80 (2013).
Sinder, B. P. et al. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 71, 115–123 (2015).
Jacobsen, C. M. et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J. Bone Miner. Res. 29, 2297–2306 (2014).
Roschger, A. et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 66, 182–188 (2014).
Perosky, J. E. et al. Single dose of bisphosphonate preserves gains in bone mass following cessation of sclerostin antibody in Brtl/+ osteogenesis imperfecta model. Bone 93, 79–85 (2016).
Willing, M. C. et al. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am. J. Hum. Genet. 55, 638–647 (1994).
Besio, R. & Forlino, A. Treatment options for osteogenesis imperfecta. Expert Opin. Orphan Drugs 3, 165–181 (2015).
Otsuru, S. et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 120, 1933–1941 (2012).
Jones, G. N. et al. Potential of human fetal chorionic stem cells for the treatment of osteogenesis imperfecta. Stem Cells Dev. 23, 262–276 (2014).
Besio, R. & Forlino, A. New frontiers for dominant osteogenesis imperfecta treatment: gene/cellular therapy approaches. Adv. Regen. Biol. 2, 27964 (2015).
Chitty, L. S. et al. EP21.04: BOOSTB4: a clinical study to determine safety and efficacy of pre- and/or postnatal stem cell transplantation for treatment of osteogenesis imperfecta. Ultrasound Obstet. Gynecol. 48 (Suppl. 1), 356 (2016).
Colige, A. et al. Human Ehlers–Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am. J. Hum. Genet. 65, 308–317 (1999).
Li, S. W. et al. Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem. J. 355, 271–278 (2001).
Weintrob, J. C. Orthotic management for children with osteogenesis imperfecta. Connect. Tissue Res. 31, S41–S43 (1995).
Colige, A. et al. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc. Natl Acad. Sci. USA 94, 2374–2379 (1997).
Bachinger, H. P., Mizuno, K., Vranka, J. & Boudko, S. P. in Comprehensive Natural Products II: Chemistry and Biology (eds Mander, L. & Liu, H.-W. ) 469–530 (Elsevier Ltd, 2010).
Acknowledgements
The authors thank M. Balasubramanian (Department of Oncology & Metabolism, University of Sheffield, Sheffield Children's NHS Foundation, UK) for providing the images shown in Figure 1.
Author information
Authors and Affiliations
Contributions
Introduction (J.C.M.); Epidemiology (P.H.B.); Mechanisms/pathophysiology (H.P.B., A.F., O.S., A.D.P., N.F.-Z., P.H.B. and J.C.M.); Diagnosis, screening and prevention (P.H.B.); Management (K.M., D.K., F.F., N.J.B. and J.C.M.); Quality of life (K.M.); Outlook (O.S., K.M.K. and A.F.); Overview of Primer (A.F. and J.C.M.).
Corresponding author
Ethics declarations
Competing interests
K.M.K. received support from Amgen (sclerostin antibody) and Mereo BioPharma (grant; sclerostin antibody) for research studies. N.J.B. received honoraria from Internis and Alexion, consultation fees (paid to the University) from Alexion, Ultragenyx, Mereo and Amgen, and research grants (paid to Sheffield Childrens NHS Foundation) from Amgen, Alexion and Merck. F.F. received royalties from PegaMedical. All other authors declare no competing interest.
Rights and permissions
About this article
Cite this article
Marini, J., Forlino, A., Bächinger, H. et al. Osteogenesis imperfecta. Nat Rev Dis Primers 3, 17052 (2017). https://doi.org/10.1038/nrdp.2017.52
Published:
DOI: https://doi.org/10.1038/nrdp.2017.52
This article is cited by
-
Echocardiographic abnormalities and joint hypermobility in Chinese patients with Osteogenesis imperfecta
Orphanet Journal of Rare Diseases (2024)
-
The impact of osteogenesis imperfecta severity on oral health-related quality of life in Spain: a cross-sectional study
Orphanet Journal of Rare Diseases (2024)
-
The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7
Cardiovascular Diabetology (2024)
-
The IMPACT survey: a mixed methods study to understand the experience of children, adolescents and adults with osteogenesis imperfecta and their caregivers
Orphanet Journal of Rare Diseases (2024)
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease
Cell Research (2024)