Abstract
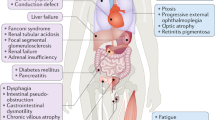
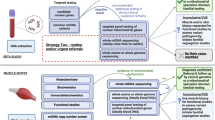
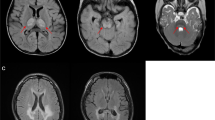
Mitochondrial diseases are a group of genetic disorders that are characterized by defects in oxidative phosphorylation and caused by mutations in genes in the nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) that encode structural mitochondrial proteins or proteins involved in mitochondrial function. Mitochondrial diseases are the most common group of inherited metabolic disorders and are among the most common forms of inherited neurological disorders. One of the challenges of mitochondrial diseases is the marked clinical variation seen in patients, which can delay diagnosis. However, advances in next-generation sequencing techniques have substantially improved diagnosis, particularly in children. Establishing a genetic diagnosis allows patients with mitochondrial diseases to have reproductive options, but this is more challenging for women with pathogenetic mtDNA mutations that are strictly maternally inherited. Recent advances in in vitro fertilization techniques, including mitochondrial donation, will offer a better reproductive choice for these women in the future. The treatment of patients with mitochondrial diseases remains a challenge, but guidelines are available to manage the complications of disease. Moreover, an increasing number of therapeutic options are being considered, and with the development of large cohorts of patients and biomarkers, several clinical trials are in progress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Duchen, M. R. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 25, 365–451 (2004).
Hopper, R. K. et al. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45, 2524–2536 (2006).
Hughes, D. A., Jastroch, M., Stoneking, M. & Klingenspor, M. Molecular evolution of UCP1 and the evolutionary history of mammalian non-shivering thermogenesis. BMC Evol. Biol. 9, 4 (2009).
Dolezal, P., Likic, V., Tachezy, J. & Lithgow, T. Evolution of the molecular machines for protein import into mitochondria. Science 313, 314–318 (2006).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
McFarland, R., Taylor, R. W. & Turnbull, D. M. A neurological perspective on mitochondrial disease. Lancet Neurol. 9, 829–840 (2010).
Hakonen, A. H. et al. Abundance of the POLG disease mutations in Europe, Australia, New Zealand, and the United States explained by single ancient European founders. Eur. J. Hum. Genet. 15, 779–783 (2007).
Lake, N. J., Compton, A. G., Rahman, S. & Thorburn, D. R. Leigh syndrome: one disorder, more than 75 monogenic causes. Ann. Neurol. 79, 190–203 (2015). This paper details the genetic, biochemical, clinical, metabolic and neuroradiological heterogeneity of Leigh syndrome, the most common childhood presentation of mitochondrial diseases, that comprises >75 monogenic disorders.
Skladal, D., Halliday, J. & Thorburn, D. R. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 126, 1905–1912 (2003).
Ryan, E. et al. Mitochondrial cytopathies, phenotypic heterogeneity and a high incidence. Ir. Med. J. 99, 262–264 (2006).
Gorman, G. S. et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759 (2015). This paper presents the most up-to-date, detailed estimates of the prevalence of adult mitochondrial diseases, and showed that the prevalence of mitochondrial diseases caused by mutations in mtDNA is estimated at 9.6 cases per 100,000 individuals and the prevalence of mitochondrial diseases caused by mutations in nDNA is estimated at 2.9 cases per 100,000 individuals.
Manwaring, N. et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion 7, 230–233 (2007).
Elliott, H. R., Samuels, D. C., Eden, J. A., Relton, C. L. & Chinnery, P. F. Pathogenic mitochondrial DNA mutations are common in the general population. Am. J. Hum. Genet. 83, 254–260 (2008).
Thorburn, D. R. Mitochondrial disorders: prevalence, myths and advances. J. Inherit. Metab. Dis. 27, 349–362 (2004).
Lebon, S. et al. Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J. Med. Genet. 40, 896–899 (2003).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Calvo, S. E. & Mootha, V. K. The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 11, 25–44 (2010).
Lapuente-Brun, E. et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570 (2013).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Andrews, R. M. et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 23, 147 (1999).
Richter, R. et al. Translation termination in human mitochondrial ribosomes. Biochem. Soc. Trans. 38, 1523–1526 (2010).
Koopman, W. J., Willems, P. H. & Smeitink, J. A. Monogenic mitochondrial disorders. N. Engl. J. Med. 366, 1132–1141 (2012).
Chinnery, P. F. Mitochondrial disorders overview. GeneReviewshttps://www.ncbi.nlm.nih.gov/books/NBK1224/ (updated 14 Aug 2014).
Kornblum, C. et al. Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease. Nat. Genet. 45, 214–219 (2013).
Zheng, L. et al. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol. Cell 32, 325–336 (2008).
Kazak, L., Reyes, A. & Holt, I. J. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 13, 659–671 (2012).
Ashley, N. et al. Defects in maintenance of mitochondrial DNA are associated with intramitochondrial nucleotide imbalances. Hum. Mol. Genet. 16, 1400–1411 (2007).
Moraes, C. T. et al. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am. J. Hum. Genet. 48, 492–501 (1991).
Nishigaki, Y., Martí, R., Copeland, W. C. & Hirano, M Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J. Clin. Invest. 111, 1913–1921 (2003).
Lightowlers, R. N., Taylor, R. W. & Turnbull, D. M. Mutations causing mitochondrial disease: what is new and what challenges remain? Science 349, 1494–1499 (2015).
Nesbitt, V. et al. The UK MRC Mitochondrial Disease Patient Cohort Study: clinical phenotypes associated with the m. 243A>G mutation — implications for diagnosis and management. J. Neurol. Neurosurg. Psychiatry 84, 936–938 (2013).
Chinnery, P. F., Elliott, H. R., Hudson, G., Samuels, D. C. & Relton, C. L. Epigenetics, epidemiology and mitochondrial DNA diseases. Int. J. Epidemiol. 41, 177–187 (2012).
Giordano, C. et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain 137, 335–353 (2014).
Bianco, A. et al. Mitochondrial DNA copy number differentiates the Leber's hereditary optic neuropathy affected individuals from the unaffected mutation carriers. Brain 139, e1 (2016).
Grady, J. P. et al. Disease progression in patients with single, large-scale mitochondrial DNA deletions. Brain 137, 323–334 (2014).
Brito, S. et al. Long-term survival in a child with severe encephalopathy, multiple respiratory chain deficiency and GFM1 mutations. Front. Genet. 6, 102 (2015).
Horvath, R. et al. Molecular basis of infantile reversible cytochrome c oxidase deficiency myopathy. Brain 132, 3165–3174 (2009).
Scaglia, F. et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics 114, 925–931 (2004).
Ferreira, M. et al. Progressive cavitating leukoencephalopathy associated with respiratory chain complex I deficiency and a novel mutation in NDUFS1. Neurogenetics 12, 9–17 (2011).
Uluc, K. et al. Leukoencephalopathy with brain stem and spinal cord involvement and high lactate: a genetically proven case with distinct MRI findings. J. Neurol. Sci. 273, 118–122 (2008).
Al-Hassnan, Z. N. et al. ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J. Med. Genet. 52, 186–194 (2015).
Janer, A. et al. RMND1 deficiency associated with neonatal lactic acidosis, infantile onset renal failure, deafness, and multiorgan involvement. Eur. J. Hum. Genet. 23, 1301–1307 (2015).
Bourdon, A. et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39, 776–780 (2007).
Seidowsky, A. et al. Renal involvement in MELAS syndrome — a series of 5 cases and review of the literature. Clin. Nephrol. 80, 456–463 (2013).
Haghighi, A. et al. Sengers syndrome: six novel AGK mutations in seven new families and review of the phenotypic and mutational spectrum of 29 patients. Orphanet J. Rare Dis. 9, 119 (2014).
Ghezzi, D. et al. Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am. J. Hum. Genet. 90, 1079–1087 (2012).
Gotz, A. et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 88, 635–642 (2011).
Wahbi, K. et al. Cardiac involvement is frequent in patients with the m. 8344A>G mutation of mitochondrial DNA. Neurology 74, 674–677 (2010).
Wedatilake, Y. et al. SURF1 deficiency: a multi-centre natural history study. Orphanet Rare Dis. 8, 96 (2013).
Huemer, M. et al. Clinical, morphological, biochemical, imaging and outcome parameters in 21 individuals with mitochondrial maintenance defect related to FBXL4 mutations. J. Inherit. Metab. Dis. 38, 905–914 (2015).
Yelverton, J. C. et al. The clinical and audiologic features of hearing loss due to mitochondrial mutations. Otolaryngol. Head Neck Surg. 148, 1017–1022 (2013).
Luo, L. F., Hou, C. C. & Yang, W. X. Nuclear factors: roles related to mitochondrial deafness. Gene 520, 79–89 (2013).
Leigh, D. Subacute necrotizing encephalomyelopathy in an infant. J. Neurol. Neurosurg. Psychiatry 14, 216–221 (1951).
Bonfante, E., Koenig, M. K., Adejumo, R. B., Perinjelil, V. & Riascos, R. F. The neuroimaging of Leigh syndrome: case series and review of the literature. Pediatr. Radiol. 46, 443–451 (2016).
Saneto, R. P., Cohen, B. H., Copeland, W. C. & Naviaux, R. K. Alpers–Huttenlocher syndrome. Pediatr. Neurol. 48, 167–178 (2013).
Tzoulis, C. et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain 129, 1685–1692 (2006).
Cohen, B. H., Chinnery, P. F. & Copeland, W. C. POLG-related disorders. GeneReviewshttps://www.ncbi.nlm.nih.gov/books/NBK26471/ (updated 18 Dec 2014).
Naviaux, R. K. & Nguyen, K. V. POLG mutations associated with Alpers' syndrome and mitochondrial DNA depletion. Ann. Neurol. 55, 706–712 (2004).
Sofou, K. et al. Whole exome sequencing reveals mutations in NARS2 and PARS2, encoding the mitochondrial asparaginyl-tRNA synthetase and prolyl-tRNA synthetase, in patients with Alpers syndrome. Mol. Genet. Genomic Med. 3, 59–68 (2015).
Elo, J. M. et al. Mitochondrial phenylalanyl-tRNA synthetase mutations underlie fatal infantile Alpers encephalopathy. Hum. Mol. Genet. 21, 4521–4529 (2012).
Rötig, A., Bourgeron, T., Chretien, D., Rustin, P. & Munnich, A. Spectrum of mitochondrial DNA rearrangements in the Pearson marrow–pancreas syndrome. Hum. Mol. Genet. 4, 1327–1330 (1995).
Rotig, A. et al. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet 1, 902–903 (1989).
Nesbitt, V., Bartlett, K., Taylor, R. W. & McFarland, R. Congenital lactic acidosis and mitochondrial disease — when to suspect and how to manage. J. Neonatal Perinatal Med. 4, 179–187 (2011).
Mancuso, M. et al. Mitochondrial DNA depletion: mutations in thymidine kinase gene with myopathy and SMA. Neurology 59, 1197–1202 (2002).
Wortmann, S. B. et al. Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat. Genet. 44, 797–802 (2012).
Mayr, J. A. et al. Lack of the mitochondrial protein acylglycerol kinase causes Sengers syndrome. Am. J. Hum. Genet. 90, 314–320 (2012).
Leber, T. Uber hereditare und congenital-angelegte Sehnervenleiden. Graefes Arch. Ophthalmol. 17, 249–291 (in German) (1871).
Macmillan, C. et al. Pedigree analysis of French Canadian families with T14484C Leber's hereditary optic neuropathy. Neurology 50, 417–422 (1998).
Yu-Wai-Man, P., Griffiths, P. G., Hudson, G. & Chinnery, P. F. Inherited mitochondrial optic neuropathies. J. Med. Genet. 46, 145–158 (2009).
Matthews, L. et al. MRI in Leber's hereditary optic neuropathy: the relationship to multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 86, 537–542 (2015).
Hudson, G. et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am. J. Hum. Genet. 81, 228–233 (2007).
Kirkman, M. A. et al. Gene–environment interactions in Leber hereditary optic neuropathy. Brain 132, 2317–2326 (2009).
Kearns, T. P. & Sayre, G. P. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch. Ophthalmol. 60, 280–289 (1958).
Holt, I. J., Harding, A. E. & Morgan-Hughes, J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331, 717–719 (1988). This paper describes the first pathogenetic mtDNA mutation.
Pavlakis, S. G., Phillips, P. C., DiMauro, S., De Vivo, D. C. & Rowland, L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann. Neurol. 16, 481–488 (1984).
Goto, Y.-I., Nonaka, I. & Horai, S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990). This paper was the first to observe the association of a heteroplasmic point mutation in the tRNALeu(UUR) gene in association with MELAS syndrome while devising a simple molecular diagnostic test.
Schon, E. A., DiMauro, S. & Hirano, M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat. Rev. Genet. 13, 878–890 (2012).
DiMauro, S. & Hirano, M. MERRF. GeneReviewshttps://www.ncbi.nlm.nih.gov/books/NBK1520/?report=printable (updated 29 Jan 2015).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Ortiz, R. G. et al. Variable retinal and neurologic manifestations in patients harboring the mitochondrial DNA 8993 mutation. Arch. Ophthalmol. 111, 1525–1530 (1993).
Rantamaki, M. T., Soini, H. K., Finnila, S. M., Majamaa, K. & Udd, B. Adult-onset ataxia and polyneuropathy caused by mitochondrial 8993T→C mutation. Ann. Neurol. 58, 337–340 (2005).
Tatuch, Y. et al. Heteroplasmic mtDNA mutation (T----G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am. J. Hum. Genet. 50, 852–858 (1992).
White, S. L. et al. Genetic counseling and prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am. J. Hum. Genet. 65, 474–482 (1999).
Sommerville, E. W., Chinnery, P. F., Gorman, G. S. & Taylor, R. W. Adult-onset Mendelian PEO associated with mitochondrial disease. J. Neuromuscul. Dis. 1, 119–133 (2014).
Hudson, G. et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 131, 329–337 (2008).
Verhoeven, K. et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot–Marie–Tooth type 2. Brain 129, 2093–2102 (2006).
Ishihara, N., Fujita, Y., Oka, T. & Mihara, K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 25, 2966–2977 (2006).
Whittaker, R. G. et al. Urine heteroplasmy is the best predictor of clinical outcome in the m. 3243A>G mtDNA mutation. Neurology 72, 568–569 (2009).
Greaves, L. C., Reeve, A. K., Taylor, R. W. & Turnbull, D. M. Mitochondrial DNA and disease. J. Pathol. 226, 274–286 (2012).
Rocha, M. C. et al. A novel immunofluorescent assay to investigate oxidative phosphorylation deficiency in mitochondrial myopathy: understanding mechanisms and improving diagnosis. Sci. Rep. 5, 15037 (2015).
Shaham, O. et al. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc. Natl Acad. Sci. USA 107, 1571–1575 (2010).
Haas, R. H. et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics 120, 1326–1333 (2007).
Nishino, I., Spinazzola, A. & Hirano, M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 283, 689–692 (1999). This paper was the first to elucidate the genetic basis of MNGIE syndrome, an autosomal recessive human disease associated with multiple deletions of skeletal muscle mtDNA, by identifying homozygous and compound-heterozygous mutations in the gene encoding thymidine phosphorylase.
Suomalainen, A. et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818 (2011). This paper reports on the identification of biomarkers for human mitochondrial diseases, including FGF21, as a potential first-line diagnostic test.
Davis, R. L. et al. Fibroblast growth factor 21 is a sensitive biomarker of mitochondrial disease. Neurology 81, 1819–1826 (2013).
Yatsuga, S. et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol. 78, 814–823 (2015).
Honzik, T. et al. Mitochondrial encephalocardio-myopathy with early neonatal onset due to TMEM70 mutation. Arch. Dis. Child. 95, 296–301 (2010).
Wortmann, S. B. et al. 3-Methylglutaconic aciduria — lessons from 50 genes and 977 patients. J. Inherit. Metab. Dis. 36, 913–921 (2013).
Carrozzo, R. et al. Succinate-CoA ligase deficiency due to mutations in SUCLA2 and SUCLG1: phenotype and genotype correlations in 71 patients. J. Inherit. Metab. Dis. 39, 243–252 (2016).
Steffann, J. et al. Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J. Med. Genet. 43, 244–247 (2006).
Craven, L. et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465, 82–85 (2010). This paper shows that the transfer of pronuclei between abnormally fertilized human zygotes yielded minimal carry-over of donor zygote mtDNA, suggesting that the prevention of transmission of human mitochondrial diseases caused by mutations in mtDNA was possible and provides women with mtDNA mutations more reproductive options.
Tachibana, M. et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461, 367–372 (2009).
Nuffied Council on Bioethics. Novel techniques for the prevention of mitochondrial DNA disorders: an ethical review. NuffieldBioEthicshttp://nuffieldbioethics.org/project/mitochondrial-dna-disorders/ (2012).
National Academies Press. Mitochondrial replacement techniques: ethical, social, and policy considerations. NAPhttp://www.nationalacademies.org/hmd/Reports/2016/Mitochondrial-Replacement-Techniques.aspx (2016).
Hyslop, L. A. et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature 534, 383–386 (2016).
Avula, S., Parikh, S., Demarest, S., Kurz, J. & Gropman, A. Treatment of mitochondrial disorders. Curr. Treat. Options Neurol. 16, 292 (2014).
Camp, K. M. et al. Nutritional interventions in primary mitochondrial disorders: developing an evidence base. Mol. Genet. Metab.http://dx.doi.org/10.1016/j.ymgme.2016.09.002 (2016).
Enns, G. M. Treatment of mitochondrial disorders: antioxidants and beyond. J. Child Neurol. 29, 1235–1240 (2014).
Rodriguez, M. C. et al. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 35, 235–242 (2007).
Panetta, J., Smith, L. J. & Boneh, A. Effect of high-dose vitamins, coenzyme Q and high-fat diet in paediatric patients with mitochondrial diseases. J. Inherit. Metab. Dis. 27, 487–498 (2004).
Matthews, P. M. et al. Coenzyme Q10 with multiple vitamins is generally ineffective in treatment of mitochondrial disease. Neurology 43, 884–884 (1993).
Marriage, B. J., Clandinin, M. T., Macdonald, I. M. & Glerum, D. M. Cofactor treatment improves ATP synthetic capacity in patients with oxidative phosphorylation disorders. Mol. Genet. Metab. 81, 263–272 (2004).
Napolitano, A. et al. Long-term treatment with idebenone and riboflavin in a patient with MELAS. Neurol. Sci. 21, S981–S9812 (2000).
Tarnopolsky, M. A., Roy, B. D. & MacDonald, J. R. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 20, 1502–1509 (1997).
Koga, Y. et al. l-Arginine improves the symptoms of strokelike episodes in MELAS. Neurology 64, 710–712 (2005).
Koga, Y. et al. MELAS and l-arginine therapy. Mitochondrion 7, 133–139 (2007).
Koenig, M. K. et al. Recommendations for the management of strokelike episodes in patients with mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes. JAMA Neurol. 73, 591–594 (2016).
Ng, Y. S. et al. Sudden adult death syndrome in m.3243A>G-related mitochondrial disease: anunrecognized clinical entity in young, asymptomatic adults. Eur. Heart J. 37, 2552–2559 (2016).
Newcastle University. Wellcome Trust centre for mitochondrial research. Newcastle Universitywww.newcastle-mitochondria.com/ (accessed 1 June 2016).
Viscomi, C. et al. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat. Med. 16, 869–871 (2010). This study demonstrates the therapeutic efficacy of metronidazole and N-acetylcysteine (with an additive effect with dual therapy) in ethylmalonic encephalopathy by substantially prolonging the lifespan of Ethe1-deficient mice in addition to marked clinical improvement in five affected children.
Tiranti, V. et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 15, 200–205 (2009).
Garone, C., Tadesse, S. & Hirano, M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain 134, 3326–3332 (2011).
Bax, B. E. et al. Clinical and biochemical improvements in a patient with MNGIE following enzyme replacement. Neurology 81, 1269–1271 (2013).
Halter, J. P. et al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain 138, 2847–2858 (2015).
Emmanuele, V. et al. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch. Neurol. 69, 978–983 (2012).
Quinzii, C. M., Emmanuele, V. & Hirano, M. Clinical presentations of coenzyme Q10 deficiency syndrome. Mol. Syndromol. 5, 141–146 (2014).
Klopstock, T. et al. Persistence of the treatment effect of idebenone in Leber's hereditary optic neuropathy. Brain 136, e230 (2013).
Klopstock, T. et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain 134, 2677–2686 (2011). This 24-week, multicentre, double-blind, randomized, placebo-controlled trial of idebenone was shown to preserve vision in patients with LHON and discordant vision at baseline, representing the first large randomized controlled therapeutic trial in a common form of mitochondrial disease.
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02023866 (2016).
Martinelli, D. et al. EPI-743 reverses the progression of the pediatric mitochondrial disease — genetically defined Leigh syndrome. Mol. Genet. Metab. 107, 383–388 (2012).
Haack, T. B. et al. Exome sequencing identifies ACAD9 mutations as a cause of complex I deficiency. Nat. Genet. 42, 1131–1134 (2010).
Ghezzi, D. et al. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 86, 639–649 (2010).
Nouws, J. et al. A patient with complex I deficiency caused by a novel ACAD9 mutation not responding to riboflavin treatment. JIMD Rep. 12, 37–45 (2014).
Thommasen, H. V. & Zhang, W. Impact of chronic disease on quality of life in the Bella Coola Valley. Rural Remote Health 6, 528 (2006).
Orsucci, D., Calsolaro, V., Siciliano, G. & Mancuso, M. Quality of life in adult patients with mitochondrial myopathy. Neuroepidemiology 38, 194–195 (2012).
Varvogli, L. & Waisbren, S. E. Personality profiles of mothers of children with mitochondrial disorders. J. Inherit. Metab. Dis. 22, 615–622 (1999).
Read, C. Y. The demands of biochemical genetic disorders: a survey of mothers of children with mitochondrial disease or phenylketonuria. J. Pediatr. Nurs. 18, 181–186 (2003).
Boles, R. G. et al. A high predisposition to depression and anxiety in mothers and other matrilineal relatives of children with presumed maternally inherited mitochondrial disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 137B, 20–24 (2005).
Kim, K. R. et al. Caregiver's burden and quality of life in mitochondrial disease. Pediatr. Neurol. 42, 271–276 (2010).
Noorda, G. et al. Mitochondrial disease: needs and problems of children, their parents and family. Asystematic review and pilot study into the need for information of parents during the diagnostic phase. J. Inherit. Metab. Dis. 30, 333–340 (2007).
Sexton, A. C., Sahhar, M., Thorburn, D. R. & Metcalfe, S. A. Impact of a genetic diagnosis of a mitochondrial disorder 5–17 years after the death of an affected child. J. Genet. Couns. 17, 261–273 (2008).
Rogac, M., Meznaric, M., Zeviani, M., Sperl, W. & Neubauer, D. Functional outcome of children with mitochondrial diseases. Pediatr. Neurol. 44, 340–346 (2011).
Kratz, L., Uding, N., Trahms, C. M., Villareale, N. & Kieckhefer, G. M. Managing childhood chronic illness: parent perspectives and implications for parent-provider relationships. Fam. Syst. Health 27, 303–313 (2009).
Department of Health. The human fertilisation and embryology (mitochondrial donation) regulations 2015. Legislationhttp://www.legislation.gov.uk/ukdsi/2015/9780111125816/impacts (accessed 1 June 2016).
Calvo, S. E. et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl Med. 4, 118ra10 (2012).
Falk, M. J. et al. Mitochondrial Disease Sequence Data Resource (MSeqDR): a global grass-roots consortium to facilitate deposition, curation, annotation, and integrated analysis of genomic data for the mitochondrial disease clinical and research communities. Mol. Genet. Metab. 114, 388–396 (2015).
Bender, A. et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38, 515–517 (2006).
Oldfors, A. et al. Mitochondrial abnormalities in inclusion-body myositis. Neurology 66, S49–S55 (2006).
Pietilainen, K. H. et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 5, e51 (2008).
Suomalainen, A. & Isohanni, P. Mitochondrial DNA depletion syndromes — many genes, common mechanisms. Neuromuscul. Disord. 20, 429–437 (2010).
Gonzalez-Vioque, E., Torres-Torronteras, J., Andreu, A. L. & Marti, R. Limited dCTP availability accounts for mitochondrial DNA depletion in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). PLoS Genet. 7, e1002035 (2011).
Nikkanen, J. et al. Mitochondrial DNA replication defects disturb cellular dNTP pools and remodel one-carbon metabolism. Cell Metab. 23, 635–648 (2016).
Camara, Y. et al. Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome. Hum. Mol. Genet. 23, 2459–2467 (2014).
Tyynismaa, H. & Schon, E. A. Mixing and matching mitochondrial aminoacyl synthetases and their tRNAs: a new way to treat respiratory chain disorders? EMBO Mol. Med. 6, 155–157 (2014).
Vafai, S. B. & Mootha, V. K. Mitochondrial disorders as windows into an ancient organelle. Nature 491, 374–383 (2012).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Nicholls, P. Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 67, 610–616 (1975).
Wai, T. et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116 (2015).
Ahola-Erkkila, S. et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 19, 1974–1984 (2010).
Schiff, M. et al. Mouse studies to shape clinical trials for mitochondrial diseases: high fat diet in Harlequin mice. PLoS ONE 6, e28823 (2011).
Kang, H. C., Lee, Y. M., Kim, H. D., Lee, J. S. & Slama, A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia 48, 82–88 (2007).
Steinfeld, R. et al. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am. J. Hum. Genet. 85, 354–363 (2009).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Pfeffer, G., Majamaa, K., Turnbull, D. M., Thorburn, D. & Chinnery, P. F. Treatment for mitochondrial disorders. Cochrane Database Syst. Rev. 4, CD004426 (2012).
Taivassalo, T. et al. Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain 129, 3391–3401 (2006).
Taivassalo, T. & Haller, R. G. Exercise and training in mitochondrial myopathies. Med. Sci. Sports Exerc. 37, 2094–2101 (2005).
Uittenbogaard, M. & Chiaramello, A. Mitochondrial biogenesis: a therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 20, 5574–5593 (2014).
Yatsuga, S. & Suomalainen, A. Effect of bezafibrate treatment on late-onset mitochondrial myopathy in mice. Hum. Mol. Genet. 21, 526–535 (2012).
Wenz, T., Diaz, F., Spiegelman, B. M. & Moraes, C. T. Activation of the PPAR/PGC-1α pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 8, 249–256 (2008).
Viscomi, C. et al. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 14, 80–90 (2011).
Cerutti, R. et al. NAD+-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 19, 1042–1049 (2014).
Khan, N. A. et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3 . EMBO Mol. Med. 6, 721–731 (2014).
Pirinen, E. et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 19, 1034–1041 (2014).
Gilkerson, R. W. et al. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum. Mol. Genet. 21, 978–990 (2012).
Johnson, S. C. et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342, 1524–1528 (2013).
Jain, I. H. et al. Hypoxia as a therapy for mitochondrial disease. Science 352, 54–61 (2016). This landmark paper shows the powerful suppressor effect of hypoxia on mitochondrial dysfunction, suggesting that the hypoxia response may serve as a potent therapeutic strategy.
Garone, C. et al. Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol. Med. 6, 1016–1027 (2014).
Hashimoto, M. et al. MitoTALEN: a general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol. Ther. 23, 1592–1599 (2015).
Minczuk, M., Papworth, M. A., Miller, J. C., Murphy, M. P. & Klug, A. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res. 36, 3926–3938 (2008).
Reddy, P. et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 161, 459–469 (2015).
Di Meo, I. et al. Effective AAV-mediated gene therapy in a mouse model of ethylmalonic encephalopathy. EMBO Mol. Med. 4, 1008–1014 (2012).
Torres-Torronteras, J. et al. Gene therapy using a liver-targeted AAV vector restores nucleoside and nucleotide homeostasis in a murine model of MNGIE. Mol. Ther. 22, 901–907 (2014).
López, L. C. et al. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum. Mol. Genet. 18, 714–722 (2009).
Dionisi-Vici, C. et al. Liver transplant in ethylmalonic encephalopathy: a new treatment for an otherwise fatal disease. Brain 139, 1045–1051 (2016).
Grabhorn, E. et al. Long-term outcomes after liver transplantation for deoxyguanosine kinase deficiency: a single-center experience and a review of the literature. Liver Transpl. 20, 464–472 (2014).
Koopman, W. J. H. et al. Mitochondrial disorders in children: toward development of small-molecule treatment strategies. EMBO Mol. Med. 8, 311–327 (2016).
Stewart, J. B. & Chinnery, P. F. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet. 16, 530–542 (2015).
Taylor, R. W. & Turnbull, D. M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402 (2005).
Darin, N., Oldfors, A., Moslemi, A. R., Holme, E. & Tulinius, M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann. Neurol. 49, 377–383 (2001).
Diogo, L. et al. Pediatric mitochondrial respiratory chain disorders in the Centro region of Portugal. Pediatr. Neurol. 40, 351–356 (2009).
Castro-Gago, M. et al. Epidemiology of pediatric mitochondrial respiratory chain disorders in northwest Spain. Pediatr. Neurol. 34, 204–211 (2006).
Uusimaa, J. et al. Childhood encephalopathies and myopathies: a prospective study in a defined population to assess the frequency of mitochondrial disorders. Pediatrics 105, 598–603 (2000).
Yatsuga, S. et al. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim. Biophys. Acta 1820, 619–624 (2012).
Pfeffer, G., Burke, A., Yu-Wai-Man, P., Compston, D. A. S. & Chinnery, P. F. Clinical features of MS associated with Leber hereditary optic neuropathy mtDNA mutations. Neurology 81, 2073–2081 (2013).
Van Goethem, G., Dermaut, B., Löfgren, A., Martin, J.-J. & Van Broeckhoven, C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 28, 211–212 (2001). This paper describes the identification of the most important nuclear gene responsible for mitochondrial diseases, and demonstrates that different mutations exhibit variable Mendelian inheritance that result in either a recessive or a dominant disorder.
Longley, M. J. et al. Mutant POLG2 disrupts DNA polymerase γ subunits and causes progressive external ophthalmoplegia. Am. J. Hum. Genet. 78, 1026–1034 (2006).
Garone, C. et al. MPV17 mutations causing adult-onset multisystemic disorder with multiple mitochondrial DNA deletions. Arch. Neurol. 69, 1648–1651 (2012).
Ronchi, D. et al. Next-generation sequencing reveals DGUOK mutations in adult patients with mitochondrial DNA multiple deletions. Brain 135, 3404–3415 (2012).
Tyynismaa, H. et al. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum. Mol. Genet. 21, 66–75 (2012).
Tyynismaa, H. et al. A heterozygous truncating mutation in RRM2B causes autosomal-dominant progressive external ophthalmoplegia with multiple mtDNA deletions. Am. J. Hum. Genet. 85, 290–295 (2009).
Spelbrink, J. N. et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 28, 223–231 (2001). This paper identified mutations in the gene encoding Twinkle, manifesting as autosomal dominant progressive external ophthalmoplegia associated with muscle-restricted multiple mtDNA deletions.
Kaukonen, J. et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289, 782–785 (2000).
Pfeffer, G. et al. Mutations in the SPG7 gene cause chronic progressive external ophthalmoplegia through disordered mitochondrial DNA maintenance. Brain 137, 1323–1336 (2014).
Gorman, G. S. et al. Clonal expansion of secondary mitochondrial DNA deletions associated with spinocerebellar ataxia type 28. JAMA Neurol. 72, 106–111 (2015).
Ronchi, D. et al. Mutations in DNA2 link progressive myopathy to mitochondrial DNA instability. Am. J. Hum. Genet. 92, 293–300 (2013).
Reyes, A. et al. RNASEH1 mutations impair mtDNA replication and cause adult-onset mitochondrial encephalomyopathy. Am. J. Hum. Genet. 97, 186–193 (2015).
Gal, A. et al. The coexistence of dynamin 2 mutation and multiple mitochondrial DNA (mtDNA) deletions in the background of severe cardiomyopathy and centronuclear myopathy. Clin. Neuropathol. 34, 89–95 (2015).
Acknowledgements
Y.K. received research support from the Japan Agency for Medical Research and Development. D.R.T. receives research support from the Australian National Health and Medical Research Council Principal Research Fellowship and by the Victorian Government's Operational Infrastructure Support Program. G.S.G., R.M.F. and D.M.T. are supported by the Wellcome Trust Centre for Mitochondrial Research, Newcastle University Centre for Ageing and Vitality (supported by the Biotechnology and Biological Sciences Research Council and the Medical Research Council (MRC)), the MRC Centre for Neuromuscular Disease, the MRC Centre for Translational Research in Neuromuscular Disease Mitochondrial Disease Patient Cohort (UK), the Lily Foundation, the UK National Institute for Health Research (NIHR) Biomedical Research Centre in Age and Age Related Diseases award to the Newcastle-upon-Tyne Hospitals NHS Foundation Trust and UK NHS Specialist Commissioners ‘Rare Mitochondrial Disorders of Adults and Children’ Service. A.S. received research support from the European Research Council, the Sigrid Jusélius Foundation and the Academy of Finland. M.Z. is supported by the MRC and a European Research Council advanced grant.
Author information
Authors and Affiliations
Contributions
Introduction (D.M.T.); Epidemiology (G.S.G. and D.R.T.); Mechanisms/pathophysiology (D.M.T., P.F.C. and M.Z.); Diagnosis, screening and prevention (G.S.G., Y.K., R.M.F. and D.R.T.); Management (S.D. and M.H.); Quality of life (D.M.T. and G.S.G.); Outlook (A.S.); Overview of Primer (G.S.G. and D.M.T.)
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Gorman, G., Chinnery, P., DiMauro, S. et al. Mitochondrial diseases. Nat Rev Dis Primers 2, 16080 (2016). https://doi.org/10.1038/nrdp.2016.80
Published:
DOI: https://doi.org/10.1038/nrdp.2016.80
This article is cited by
-
Interleukin-6-elicited chronic neuroinflammation may decrease survival but is not sufficient to drive disease progression in a mouse model of Leigh syndrome
Journal of Inflammation (2024)
-
Mechanisms and regulation of human mitochondrial transcription
Nature Reviews Molecular Cell Biology (2024)
-
The expanding diagnostic toolbox for rare genetic diseases
Nature Reviews Genetics (2024)
-
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
Nature (2024)
-
Atypical presentation of Pearson syndrome in an infant with suspected myelodysplastic syndrome
Pediatric Nephrology (2024)