Abstract
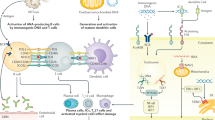
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect many organs, including the skin, joints, the central nervous system and the kidneys. Women of childbearing age and certain racial groups are typically predisposed to developing the condition. Rare, inherited, single-gene complement deficiencies are strongly associated with SLE, but the disease is inherited in a polygenic manner in most patients. Genetic interactions with environmental factors, particularly UV light exposure, Epstein–Barr virus infection and hormonal factors, might initiate the disease, resulting in immune dysregulation at the level of cytokines, T cells, B cells and macrophages. Diagnosis is primarily clinical and remains challenging because of the heterogeneity of SLE. Classification criteria have aided clinical trials, but, despite this, only one drug (that is, belimumab) has been approved for use in SLE in the past 60 years. The 10-year mortality has improved and toxic adverse effects of older medications such as cyclophosphamide and glucocorticoids have been partially offset by newer drugs such as mycophenolate mofetil and glucocorticoid-sparing regimes. However, further improvements have been hampered by the adverse effects of renal and neuropsychiatric involvement and late diagnosis. Adding to this burden is the increased risk of premature cardiovascular disease in SLE together with the risk of infection made worse by immunosuppressive therapy. Challenges remain with treatment-resistant disease and symptoms such as fatigue. Newer therapies may bring hope of better outcomes, and the refinement to stem cell and genetic techniques might offer a cure in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Faurschou, M., Starklint, H., Halberg, P. & Jacobsen, S. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. J. Rheumatol. 33, 1563–1569 (2006).
Sullivan, K. E. Genetics of systemic lupus erythematosus: clinical implications. Rheum. Dis. Clin. North Am. 26, 1229–1256 (2000).
Moser, K. L., Kelly, J. A., Lessard, C. J. & Harley, J. B. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 10, 373–379 (2009).
Tsokos, G. C. & Kammer, G. M. Molecular aberrations in human systemic lupus erythematosus. Mol. Med. Today 6, 418–424 (2000).
Deng, Y. & Tsao, B. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6, 683–692 (2010).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann. Intern. Med. 142, 953–962 (2005). SLE is more common in women than in men, suggesting that hormonal factors might be important in SLE pathogenesis. This study found an increased risk of mild-to-moderate, but not severe, SLE flare with hormone-replacement therapy compared with placebo; this is a clinically useful finding.
Chang, D. M., Lan, J. L., Lin, H. Y. & Luo, S. F. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 2924–2927 (2002).
Tektonidou, M. G., Laskari, K., Panagiotakos, D. B. & Moutsopoulos, H. M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 61, 2929–2936 (2009). This study examined risk factors for thrombosis in SLE. Significant risk factors included positive lupus anticoagulant and anticardiolipin antibody profiles. Aspirin and hydroxychloroquine had a protective role against thrombosis in antiphospholipid antibody-positive SLE, suggesting that these are clinically useful drugs.
Mok, C. C., Tang, S., To, C. & Petri, M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum. 52, 2774–2782 (2005).
Mak, A., Cheung, M. W. L., Chiew, H. J., Liu, Y. & Ho, R.C. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin. Arthritis Rheum. 41, 2830–2839 (2012).
Quismorio, F. P. & Torralba, T. P. in Dubois Lupus Erythematosus and Related Syndromes (eds Wallace, D. J. & Hahn, B. H. ) 526–540 (2013).
Castrejon, I. et al. Indices to assess patients with systemic lupus erythematosus in clinical trials, long-term observational studies, and clinical care. Clin. Exp. Rheumatol. 32, S585–S595 (2014).
Kumar, K., Chambers, S. & Gordon, C. Challenges of ethnicity in SLE. Best. Pract. Res. Clin. Rheumatol. 23, 549–561 (2009).
Pons-Estel, G. J., Alarcon, G. S., Scofield, L., Reinlib, L. & Cooper, G. S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 39, 257–268 (2010).
Lim, S. S. et al. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol. 66, 357–368 (2014).
Somers, E. C. et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 66, 369–378 (2014).
Laustrup, H., Voss, A., Green, A. & Junker, P. Occurrence of systemic lupus erythematosus in a Danish community: an 8-year prospective study. Scand. J. Rheumatol. 38, 128–132 (2009).
Rees, F. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann. Rheum. Dis. 75, 136–141 (2016).
Johnson, A. E., Gordon, C., Palmer, R. G. & Bacon, P. A. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 38, 551–558 (1995).
Yee, C. S. et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology (Oxford) 54, 836–843 (2015).
Sexton, D. J. et al. ESRD from lupus nephritis in the United States, 1995–2010. Clin. J. Am. Soc. Nephrol. 10, 251–259 (2015).
Flower, C., Hennis, A. J., Hambleton, I. R., Nicholson, G. D. & Liang, M. H. Systemic lupus erythematosus in an African Caribbean population: incidence, clinical manifestations, and survival in the Barbados National Lupus Registry. Arthritis Care Res. (Hoboken) 64, 1151–1158 (2012).
Ferucci, E. D. et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska native people, 2007–2009. Arthritis Rheumatol. 66, 2494–2502 (2014).
Segasothy, M. & Phillips, P. A. Systemic lupus erythematosus in Aborigines and Caucasians in central Australia: a comparative study. Lupus 10, 2439–2444 (2001).
Bossingham, D. Systemic lupus erythematosus in the far north of Queensland. Lupus 12, 327–331 (2003).
Yeh, K. W., Yu, C. H., Chan, P. C., Horng, J. T. & Huang, J. L. Burden of systemic lupus erythematosus in Taiwan: a population-based survey. Rheumatol. Int. 33, 1805–1811 (2013).
Ju, J. H. et al. Prevalence of systemic lupus erythematosus in South Korea: an administrative database study. J. Epidemiol. 24, 1295–1303 (2014).
Shim, J. S., Sung, Y. K., Joo, Y. B., Lee, H. S. & Bae, S. C. Prevalence and incidence of systemic lupus erythematosus in South Korea. Rheumatol. Int. 34, 909–917 (2014).
Yurkovich, M., Vostretsova, K., Chen, W. & Avina-Zubieta, J. A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. (Hobeken) 66, 608–616 (2014). This meta-analysis of published data from the inception of Medline and EMBASE databases to 2011 showed all-cause mortality was threefold higher in patients with SLE than in the general population. The highest mortality risk was with renal SLE.
Mok, C. C., Kwok, R. C. & Yip, P. S. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 65, 2154–2160 (2013).
Gonzalez, L. A., Toloza, S. M. & Alarcon, G. S. Impact of race and ethnicity in the course and outcome of systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 40, 2433–2438 (2014). This paper provides a useful overview of how SLE varies in its impact in different races, with non-white races having higher mortality, higher hospital admission rates and higher post-discharge mortality than white races. Poverty and socioeconomic status seem to be important factors in this racial variation.
Gustafsson, J. T. et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res. Ther. 14, R46 (2012).
Crow, M. K., Olferiev, M. & Kirou, K. A. Targeting of type I interferon in systemic autoimmune diseases. Transl Res. 165, 296–305 (2015).
James, J. A. Clinical perspectives on lupus genetics: advances and opportunities. Rheum. Dis. Clin. North Am. 40, 413–432 (2014).
Truedsson, L. et al. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40, 560–566 (2007).
Graham, R. R. et al. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur. J. Hum. Genet. 15, 823–830 (2007).
Price, P. et al. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol. Rev. 167, 257–274 (1999).
Santer, D. M. et al. C1q deficiency leads to the defective suppression of IFN-α in response to nucleoprotein containing immune complexes. J. Immunol. 185, 4738–4749 (2010).
Crow, Y. J. & Manel, N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 4429–4440 (2015).
Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 12, 270–279 (2011).
Niewold, T. B. et al. Association of the IRF5 risk haplotype with high serum interferon-α activity in systemic lupus erythematosus patients. Arthritis Rheum. 58, 2481–2487 (2008).
Taylor, K. E. et al. Risk alleles for systemic lupus erythematosus in a large case–control collection and associations with clinical subphenotypes. PLoS Genet. 7, e1001311 (2011). This study of 1,919 patients with SLE calculated a genetic risk score determined by the number of risk alleles. This study proposed three groups of patients with SLE: those with cumulative, single or no known associations with currently known SLE loci.
Li, Q. Z. et al. The lupus-susceptibility gene kallikrein downmodulates antibody-mediated glomerulonephritis. Genes Immun. 10, 2503–2508 (2009).
Scofield, R. H. et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 58, 2511–2517 (2008).
Costenbader, K. H., Feskanich, D., Stampfer, M. J. & Karlson, E. W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 56, 1251–1262 (2007).
Kang, I. et al. Defective control of latent Epstein–Barr virus infection in systemic lupus erythematosus. J. Immunol. 172, 1287–1294 (2004).
Yadav, P. et al. Antibodies elicited in response to EBNA-1 may cross-react with dsDNA. PLoS ONE 6, e14488 (2011).
Gorelik, G. et al. Impaired T cell protein kinase Cδ activation decreases ERK pathway signaling in idiopathic and hydralazine-induced lupus. J. Immunol. 179, 5553–5563 (2007).
Du, J. et al. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 16, 5519–5532 (2015).
Costenbader, K. H. et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 50, 849–857 (2004).
Finckh, A. et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum. 54, 3648–3654 (2006).
Stetson, D. B. Endogenous retroelements and autoimmune disease. Curr. Opin. Immunol. 24, 692–697 (2012).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Crow, M. K., Kirou, K. A. & Wohlgemuth, J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 36, 2481–2490 (2003).
Lovgren, T. et al. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Kirou, K. A. et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 1417–1428 (2006).
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Oliveira, L. et al. Dysregulation of antiviral helicase pathways in systemic lupus erythematosus. Front. Genet. 5, 418 (2014).
Gao, D. et al. Activation of cyclic GMP–AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Crispin, J. C., Kyttaris, V. C., Terhorst, C. & Tsokos, G. C. T cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 317–325 (2010).
Ettinger, R. et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J. Immunol. 178, 2872–2882 (2007).
Koshy, M., Berger, D. & Crow, M. K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Invest. 98, 2826–2837 (1996).
Nambiar, M. P. et al. Reconstitution of deficient T cell receptor ζ chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 48, 1948–1955 (2003).
Gergely, P. Jr et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 46, 175–190 (2002).
Coit, P. et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. J. Autoimmun. 43, 78–84 (2013). This study characterizes genome methylation in samples from patients with SLE and demonstrates that type I IFN-regulated genes comprise the majority of hypomethylated genes.
Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62, 234–244 (2010).
McKinney, E. F. et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 16, 586–591 (2010).
Xing, Q. et al. Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol. Int. 32, 949–958 (2012).
Lieberman, L. A. & Tsokos, G. C. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J. Biomed. Biotechnol. 2010, 740619 (2010).
Kil, L. P. et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012).
Mackay, M. et al. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J. Exp. Med. 203, 2157–2164 (2006).
Avalos, A. M. et al. Differential cytokine production and bystander activation of autoreactive B cells in response to CpG-A and CpG-B oligonucleotides. J. Immunol. 183, 6262–6268 (2009).
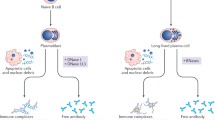
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011). The inappropriate production of autoantibodies is key to many autoimmune diseases including SLE. Although current therapies can deplete the number of B cells, long-lived plasma cells are refractory to this treatment, can propagate autoimmunity and are an important future therapeutic target.
Jacobi, A. M. et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 305–308 (2010).
Zhu, H. et al. Autoantigen microarray for high-throughput autoantibody profiling in systemic lupus erythematosus. Genomics Proteomics Bioinformatics 13, 210–218 (2015).
Chen, Y. et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J. Immunol. 183, 1346–1359 (2009).
Liu, S. et al. Ongoing immunoglobulin class switch DNA recombination in lupus B cells: analysis of switch regulatory regions. Autoimmunity 37, 1431–1443 (2004).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Podolska, M. J. et al. Inflammatory etiopathogenesis of systemic lupus erythematosus: an update. J. Inflamm. Res. 8, 1161–1171 (2015).
Golbus, J. & McCune, W. J. Lupus nephritis. Classification, prognosis, immunopathogenesis, and treatment. Rheum. Dis. Clin. North Am. 20, 213–242 (1994).
Clynes, R., Dumitru, C. & Ravetch, J. V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 (1998).
Knight, J. S. & Kaplan, M. J. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr. Opin. Rheumatol. 24, 441–450 (2012).
Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J. Immunol. 186, 4994–5003 (2011).
Hsieh, C. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. (Hoboken) 63, 5865–5874 (2011).
Deng, G. M. & Tsokos, G. C. Pathogenesis and targeted treatment of skin injury in SLE. Nat. Rev. Rheumatol. 11, 663–669 (2015).
Kaplan, M. J. Premature vascular damage in systemic lupus erythematosus. Autoimmunity 42, 580–586 (2009).
McMahon, M. et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 60, 2428–2437 (2009).
Lewis, M. J. et al. Improved monitoring of clinical response in systemic lupus erythematosus by longitudinal trend in soluble vascular cell adhesion molecule-1. Arthritis Res. Ther. 18, 5 (2016).
Gluhovschi, C. et al. What is the significance of HLA-DR antigen expression in the extraglomerular mesangium in glomerulonephritis? Hum. Immunol. 73, 1098–1101 (2012).
Achtman, J. C. & Werth, V. P. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res. Ther. 17, 182 (2015).
Liao, R. et al. Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS ONE 10, e0132724 (2015).
Arnold, W. J. (ed.) American Rheumatism Association Glossary Committee: Dictionary of the Rheumatic Diseases. Vol I: Signs and Symptoms (American College of Rheumatology, 1982).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Aggarwal, R. et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. (Hoboken) 67, 891–897 (2015).
Gladman, D. D., Ibanez, D. & Urowitz, M. B. Systemic Lupus Erythematosus Disease Activity Index 2000. J. Rheumatol. 29, 288–291 (2002).
Urowitz, M. B. et al. American College of Rheumatology criteria at inception, and accrual over 5 years in the SLICC inception cohort. J. Rheumatol. 41, 875–880 (2014).
Ines, L. et al. Classification of systemic lupus erythematosus: Systemic Lupus International Collaborating Clinics Versus American College of Rheumatology Criteria. A comparative study of 2,055 patients from a real-life, international systemic lupus erythematosus cohort. Arthritis Care Res. (Hoboken) 67, 1180–1185 (2015).
Amezcua-Guerra, L. M., Higuera-Ortiz, V., Arteaga-García, U., Gallegos-Nava, S. & Hübbe-Tena, C. Performance of the 2012 Systemic Lupus International Collaborating Clinics and the 1997 American College of Rheumatology classification criteria for systemic lupus erythematosus in a real-life scenario. Arthritis Care Res. (Hoboken) 67, 437–441 (2015).
Touma, Z., Gladman, D. D. & Urowitz, M. B. in Dubois Lupus Erythematosus and Related Syndromes (eds Wallace, D. J. & Hahn, B. H. ) 563–581 (2013).
Abu-Shakra, M. et al. Mortality studies in systemic lupus erythematosus. Results from a single center. II. Predictor variables for mortality. J. Rheumatol. 22, 1265–1270 (1995).
Steiman, A. J. et al. Prolonged clinical remission in patients with systemic lupus erythematosus. J. Rheumatol. 41, 1808–1816 (2014).
Steiman, A. J. et al. Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J. Rheumatol. 37, 1822–1827 (2010).
Franklyn, K. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis.http://dx.doi.org/10.1136/annrheumdis-2015-207726 (2015).
Gladman, D. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 39, 363–369 (1996).
Gladman, D. D. et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 40, 809–813 (1997).
Rahman, P. et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 10, 93–96 (2001).
Bruce, I. N. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann. Rheum. Dis. 74, 1706–1713 (2015). This is a multinational study of 1,722 patients with SLE that found that patients with tissue damage are at higher risk of further damage accrual, earlier mortality and worse physical functioning. The identification of these patients represents a key strategy in the future and may be predicted by use of the SDI.
Gladman, D. D. et al. Recommendations for frequency of visits to monitor systemic lupus erythematosus in asymptomatic patients: data from an observational cohort study. J. Rheumatol. 40, 630–633 (2013).
Mosca, L. et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women — 2011 update: a guideline from the American Heart Association. J. Am. Coll. Cardiol. 57, 1404–1423 (2011).
Urowitz, M. B., Ibanez, D. & Gladman, D. D. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J. Rheumatol. 34, 70–75 (2007).
Tunnicliffe, D. J. et al. Diagnosis, monitoring and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res. (Hoboken) 67, 1440–1453 (2015). Although management guidelines for SLE have been published by several key groups, this systematic analysis suggests that there are substantial disparities between guidelines and the need for more international consensus in the management of patients with SLE. There is a need for understudied areas of SLE to be better represented in future guidelines.
Mosca, M. et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann. Rheum. Dis. 69, 1269–1274 (2010).
Bultink, I. E. Osteoporosis and fractures in systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 64, 72–78 (2012).
Jeltsch-David, H. & Muller, S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat. Rev. Neurol. 10, 579–596 (2014).
Ibanez, D. et al. Optimal frequency of visits for patients with systemic lupus erythematosus to measure disease activity over time. J. Rheumatol. 38, 60–63 (2011).
Pego-Reigosa, J. M. et al. Efficacy and safety of nonbiologic immunosuppressants in the treatment of nonrenal systemic lupus erythematosus: a systematic review. Arthritis Care Res. (Hoboken) 65, 1775–1785 (2013).
Alarcon, G. S. et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 66, 1168–1172 (2007).
Tsakonas, E. et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus 7, 1180–1185 (1998).
Chen, Y.-M. et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population based cohort study. Rheumatology (Oxford) 54, 1244–1249 (2015).
van Vollenhoven, R. F. et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann. Rheum. Dis. 71, 1343–1349 (2012).
Wallace, D. J. et al. Safety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosus. Lupus 22, 1144–1154 (2013).
Petri, M. A. et al. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 50, 2858–2568 (2004).
van Vollenhoven, R. F. & McGuire, J. L. Estrogen, progesterone, and testosterone: can they be used to treat autoimmune diseases? Cleve. Clin. J. Med. 61, 2276–2284 (1994).
Naafs, B. et al. Thalidomide treatment of subacute cutaneous lupus erythematosus. Br. J. Dermatol. 107, 83–86 (1982).
Wolfe, F. et al. Fibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activity. J. Rheumatol. 36, 82–88 (2009).
Appenzeller, S., Pallone, A. T., Natalin, R. A. & Costallat, L. T. Prevalence of thyroid dysfunction in systemic lupus erythematosus. J. Clin. Rheumatol. 15, 117–119 (2009).
Gladman, D. et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J. Rheumatol. 30, 1955–1959 (2003).
Thamer, M. et al. Prednisone, lupus activity and permanent organ damage. J. Rheumatol. 36, 560–564 (2009).
Chambers, S. et al. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 48, 673–675 (2009).
Fischer-Betz, R. et al. Renal outcome in patients with lupus nephritis using a steroid-free regimen of monthly intravenous cyclophosphamide: a prospective observational study. J. Rheumatol. 39, 2211–2217 (2012).
Zeher, M. et al. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus 20, 1484–1493 (2011).
Condon, M. B. et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann. Rheum. Dis. 72, 1280–1286 (2013). Although evidence from case series suggested that B cell depletion with rituximab had benefits in SLE nephritis, the LUNAR and EXPLORER trials did not meet end points. This open-label study demonstrates that rituximab and low-dose glucocorticoids in class IV SLE nephritis can be effective treatments.
Tak, M. Treatment of severe lupus nephritis: the new horizon. Nat. Rev. Nephrol. 11, 46–61 (2015). Historically, cyclophosphamide with its attendant adverse effects had been the mainstay of treatment for patients with SLE nephritis. This review demonstrates the progression to increased use of mycophenolate mofetil as induction therapy, owing to its efficacy at inducing remission compared with lupus nephritis, with a better safety profile than cyclophosphamide.
Houssiau, F. A. et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 46, 2121–2131 (2002).
Houssiau, F. A. et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann. Rheum. Dis. 69, 61–64 (2010).
Gunnarsson, I. & Jonsdottir, T. Rituximab treatment in lupus nephritis — where do we stand? Lupus 22, 381–389 (2013).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
van Vollenhoven, R. F. Rituximab — shadow, illusion or light? Autoimmun. Rev. 11, 563–567 (2012).
US National Library of Medicine. RING — rituximab for lupus nephritis with remission as a goal (RING). ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01673295 (2012).
Dooley, M. A. et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 365, 1886–1895 (2011).
Houssiau, F. A. et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis trial. Ann. Rheum. Dis. 69, 2083–2089 (2010).
Pons-Estel, G. et al. Therapeutic plasma exchange for the management of refractory systemic autoimmune diseases: report of 31 cases and review of the literature. Autoimmun. Rev. 10, 679–684 (2011).
Schmeding, A. & Schneider, M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Prac. Res. Clin. Rheumatol. 27, 363–375 (2013).
Yelin, E. et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 57, 356–363 (2007).
Gordon, C. et al. The substantial burden of systemic lupus erythematosus on the productivity and careers of patients: a European patient-driven online survey. Rheumatology (Oxford) 52, 2292–2301 (2013).
Yazdany, J. Health-related quality of life measurement in adult systemic lupus erythematosus: Lupus Quality of Life (LupusQoL), Systemic Lupus Erythematosus-Specific Quality of Life Questionnaire (SLEQOL), and Systemic Lupus Erythematosus Quality of Life Questionnaire (L-QoL). Arthritis Care Res. (Hoboken) 63, S2413–S2419 (2011).
Gladman, D. et al. Systemic Lupus International Collaborating Clinics conference on assessment of lupus flare and quality of life measures in SLE. Systemic Lupus International Collaborating Clinics Group. J. Rheumatol 23, 1953–1955 (1996).
Dua, A. B. et al. Top 10 recent developments in health-related quality of life in patients with systemic lupus erythematosus. Curr. Rheumatol. Rep. 15, 380 (2013).
Ahn, G. E. & Ramsey-Goldman, R. Fatigue in systemic lupus erythematosus. Int. J. Clin. Rheumtol. 7, 217–227 (2012). This paper is an overview of the factors that are important in SLE fatigue. Although obesity, physical activity levels, depression, anxiety and vitamin D levels are important, the relationship to disease activity levels is much less clear.
Ruiz-Irastorza, G., Gordo, S., Olivares, N., Egurbide, M.-V. & Aguirre, C. Changes in vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damage. Arthritis Care Res. (Hoboken) 62, 1160–1165 (2010).
Furie, R. et al. Clinical, laboratory and health-related quality of life correlates of Systemic Lupus Erythematosus Responder Index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci. Med. 1, e000031 (2014).
Hanly, J. G. et al. Mood disorders in systemic lupus erythematosus: results from an international, inception cohort study. Arthritis Rheum. 67, 1837–1847 (2015).
Ruiz-Arruza, I. et al. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology (Oxford) 53, 1470–1476 (2014). Using an observational cohort of 230 patients with SLE at inception with a 5-year follow-up and SLICC as a measure of tissue damage, 37% of patients had accrued damage. Doses of >7.5 mg daily of glucocorticoids were associated with higher levels of damage attributable to the drug.
Scofield, L., Reinlib, L., Alarcón, G. S. & Cooper, G. S. Employment and disability issues in systemic lupus erythematosus: a review. Arthritis Care Res. (Hoboken) 59, 1475–1479 (2008).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
James, J. A. et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 16, 1401–1409 (2007).
Doria, A. et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am. J. Med. 119, 700–706 (2006).
Abd-Elkareem, M. I., Al Tamimy, H. M., Khamis, O. A., Abdellatif, S. S. & Hussein, M. R. Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: preliminary findings. Clin. Exp. Nephrol. 14, 548–557 (2010).
Xuejing, Z. et al. Urinary TWEAK level as a marker of lupus nephritis activity in 46 cases. J. Biomed. Biotechnol. 2012, 359647 (2012).
Lapteva, L. et al. Anti-N-methyl-d-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 54, 2505–2514 (2006).
Kowal, C. et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA 103, 19854–19859 (2006).
Urowitz, M. B. et al. The bimodal mortality pattern of systemic lupus erythematosus. Am. J. Med. 60, 19221–19225 (1976)
Magder, L. S. & Petri, M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am. J. Epidemiol. 176, 708–719 (2012).
Esdaile, J. M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 44, 2331–2337 (2001).
Bertsias, G. et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann. Rheum. Dis. 67, 195–120 (2008).
Lai, C. H. et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann. Rheum. Dis.http://dx.doi.org/10.1136/annrheumdis-2015-207719 (2015).
Myasoedova, E. et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 70, 482–487 (2011).
van Vollenhoven, R. F. et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann. Rheum. Dis. 73, 958–967 (2014).
Drenkard, C. et al. Remission of systematic lupus erythematosus. Medicine 75, 88–98 (1996).
Nossent, J. et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus 19, 949–956 (2010).
Zen, M. et al. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann. Rheum. Dis. 74, 2117–2122 (2015).
Urowitz, M. B. et al. Prolonged remission in systemic lupus erythematosus. J. Rheumatol 8, 1467–1472 (2005).
Mosca, M. et al. Treat-to-target in systemic lupus erythematosus: where are we today? Clin. Exp. Rheum. 30, S112–S115 (2012).
US FDA. Guidance for Industry. Systemic Lupus Erythematosus — Developing Medical Products for Treatment (FDA, 2010)
Wallace, D. J. et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann. Rheum. Dis. 73, 183–190 (2014).
Clowse, M. E. B. et al. Efficacy safety epratuzumab patients with moderate-to-severe system. lupus erythematosus: results from two phase 3 randomized, placebo-controlled trials. Arthritis Rheumatol. Abstr. 67 (Suppl. 10), 4L (2015).
US National Library of Medicine. Rituximab and belimumab for lupus nephritis. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02260934?term=NCT02260934&rank=1 (2014).
Koenen, H. J. et al. A novel bispecific antihuman CD40/CD86 fusion protein with T-cell tolerizing potential. Transplantation 78, 1429–1438 (2004).
Kyttaris, V. C., Juang, Y.-T. & Tsokos, G. C. Gene therapy in systemic lupus erythematosus. Lupus 13, 353–358 (2004).
Collins, E. & Gilkeson, G. Hematopoetic and mesenchymal stem cell transplantation in the treatment of refractory systemic lupus erythematosus — where are we now? Clin. Immunol. 148, 328–334 (2013). This paper is an overview of the challenges facing stem cell transplantation for SLE. Only refractory patients with severe disease have been offered stem cell procedures and this has meant that mortality from the procedure remains high. The use of different conditioning regimes and non-myeloablative mesenchymal stem cells may be an important factor in promoting the success of these regimes in future.
Bombardier, C., Gladman, D. D., Urowitz, M. B., Caron, D. & Chang, C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 35, 630–640 (1992).
Touma, Z. et al. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus 20, 67–70 (2011).
Petri, M. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558 (2005).
Symmons, D. P. et al. Development and assessment of a computerized index of clinical disease activity in systemic lupus erythematosus. Members of the British Isles Lupus Assessment Group (BILAG). Q. J. Med. 69, 927–937 (1988).
Isenberg, D. A. et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 902–906 (2005).
Touma, Z., Gladman, D. D., Ibañez, D. & Urowitz, M.B. Development and initial validation of the Systemic Lupus Erythematosus Disease Activity Index 2000 responder index 50. J. Rheumatol. 38, 275–284 (2011).
Furie, R. A. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 61, 1143–1151 (2009).
Ibanez, D., Gladman, D. D. & Urowitz, M. Summarizing disease features over time: II. Variability measures of SLEDAI-2K. J. Rheumatol. 34, 336–340 (2007).
Li, Z.-G., Mu, R., Dai, Z.-P. & Gao, X.-M. T-cell vaccination in systemic lupus erythematosus with autologous activated T-cells. Lupus 14, 884–889 (2005).
Ponticelli, C. & Moroni, G. Monoclonal antibodies for systemic lupus erythematosus (SLE). Pharmaceuticals 3, 300–322 (2010).
Isenberg, D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 (2015).
Furie, R. A. et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann. Rheum. Dis. 74, 1667–1675 (2015).
Isenberg, D. et al. Efficacy and safety of tabalumab in patients with systemic lupus erythematosus (SLE): results from 2 phase 3, 52-week, multicenter, randomized, placebo-controlled trials. 74, 141 (2015).
Alarcon-Segovia, D. et al. LJP 394 for the prevention of renal flare in patients with systemic lupus erythematosus. Arthritis Rheum. 48, 442–454 (2003).
Aringer, M. et al. Adverse events and efficacy of anti-TNF-α blockade with infliximab in patients with systemic lupus erythematosus: long term follow up of 13 patients. Rheumatology (Oxford) 48, 1451–1454 (2009).
Ostendorf, B. et al. Preliminary results of safety and efficacy of the interleukin-1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann. Rheum. Dis. 64, 630–633 (2005).
Wallace, D. J. et al. Improvement of disease activity and reduction of severe flares following subcutaneous administration of IL-6 monoclonal antibody (mAb) in subjects with active generalized systemic lupus erythematosus (SLE). ACRhttp://acrabstracts.org/abstract/improvement-of-disease-activity-and-reduction-of-severe-flares-following-subcutaneous-administration-of-an-il-6-monoclonal-antibody-mab-in-subjects-with-active-generalized-systemic-lupus-erythematos/ (2014).
Llorente, L. et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 43, 1790–1800 (2000).
Khamashta, M. et al. Safety efficacy sifalimumab, anti IFN-α monoclonal antibody, phase 2b study moderate severe system. lupus erythematosus (SLE). Arthritis Rheum. Abstr. 66, S312 (2014).
Furie, R. et al. Anifrolumab, an anti-interferon α receptor monoclonal antibody, in moderate to severe systemic lupus erythematosus (SLE). Arthritis Rheumatol. Abstr. 67 (Suppl. 10), 3223 (2015).
Omdal, R. et al. Fatigue in patients with systemic lupus erythematosus: the psychosocial aspects. J. Rheumatol. 30, 283–287 (2003).
Ad hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis Rheum. 57, 1348–1357 (2007).
Hanly, J. G., Omisade, A., Su, L., Farewell, V. & Fisk, J. D. ANAM: automated psychological assessment metrics assessment of cognitive function in systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis by computerized neuropsychological tests. Arthritis Rheum. 62, 1478–1486 (2010).
Panopalis, P. et al. The systemic lupus erythematosus tri-nation study: longitudinal changes in physical and mental well-being. Rheumatology (Oxford) 44, 751–755 (2005).
Fortin, P. R. et al. Impact of disease activity and cumulative damage on the health of lupus patients. Lupus 7, 101–107 (1998).
Alarcon, G. S. et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self- reported health-related quality of life early in the disease course. Arthritis Rheum. 51, 465–474 (2004).
Fernandez, M. et al. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US Cohort. Arthritis Rheum. 57, 986–992 (2007).
Bertoli, A. M. et al. Systemic lupus erythematosus in a multiethnic US cohort LUMINA (XLI): factors predictive of self-reported work disability. Ann. Rheum. Dis. 66, 12–17 (2007).
Baker, K. & Pope, J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology (Oxford) 48, 281–284 (2009).
Panopalis, P., Petri, M., Manzi, S. & the Tri-Nation Study Group. The Systemic Lupus Erythematosus Tri-Nation Study: cumulative indirect costs. Arthritis Rheum. 57, 64–70 (2007).
Clarke, A. E., Urowitz, M. B., Monga, N. & Hanly, J. G. Costs associated with severe and nonsevere systemic lupus erythematosus in Canada. Arthritis Care Res. (Hoboken) 67, 431–436 (2015).
Author information
Authors and Affiliations
Contributions
Introduction (A.K. and G.H.); Epidemiology (C.G.); Mechanisms/pathophysiology (M.K.C.); Diagnosis, screening and prevention (Z.T. and M.B.U.); Management (R.v.V.); Quality of life (G.R.-I.); Outlook (A.K.); Overview of Primer (A.K.).
Corresponding author
Ethics declarations
Competing interests
A.K. has received honoraria for speaking as well as research grants from Janssen, Celgene, Pfizer and Wyeth. C.G. has received consulting fees and/or honoraria from Bristol-Myers Squibb (BMS), GlaxoSmithKline (GSK), Lilly, Merck Serono, Parexel and UCB and grant support from UCB. R.v.V. has received research support or grants from AbbVie, Amgen, BMS, GSK, Pfizer, Roche and UCB and consulting fees or honoraria from AbbVie, Biotest, BMS, Celgene, Crescendo, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, UCB and Vertex. M.K.C. has received consulting fees or research support from AstraZeneca, BMS, GSK, Lilly, MedImmune, Novartis, Novo Nordisk and Pfizer. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kaul, A., Gordon, C., Crow, M. et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2, 16039 (2016). https://doi.org/10.1038/nrdp.2016.39
Published:
DOI: https://doi.org/10.1038/nrdp.2016.39
This article is cited by
-
Potential role of RhoA GTPase regulation in type interferon signaling in systemic lupus erythematosus
Arthritis Research & Therapy (2024)
-
Efficacy of lifestyle interventions in the management of systemic lupus erythematosus: a systematic review of the literature
Rheumatology International (2024)
-
Association between type 1 diabetes and systemic lupus erythematosus: a Mendelian randomization study
Clinical Rheumatology (2024)
-
The prevalence of neutropenia and association with infections in patients with systemic lupus erythematosus: a Swedish single-center study conducted over 14 years
Rheumatology International (2024)
-
Does baricitinib reduce disease activity in patients with systemic lupus erythematosus? A systematic review and meta-analysis of randomized controlled trials
Clinical Rheumatology (2024)