Abstract
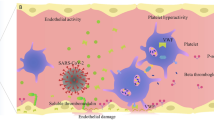
Disseminated intravascular coagulation (DIC) is an acquired syndrome characterized by widespread intravascular activation of coagulation that can be caused by infectious insults (such as sepsis) and non-infectious insults (such as trauma). The main pathophysiological mechanisms of DIC are inflammatory cytokine-initiated activation of tissue factor-dependent coagulation, insufficient control of anticoagulant pathways and plasminogen activator inhibitor 1-mediated suppression of fibrinolysis. Together, these changes give rise to endothelial dysfunction and microvascular thrombosis, which can cause organ dysfunction and seriously affect patient prognosis. Recent observations have pointed to an important role for extracellular DNA and DNA-binding proteins, such as histones, in the pathogenesis of DIC. The International Society on Thrombosis and Haemostasis (ISTH) established a DIC diagnostic scoring system consisting of global haemostatic test parameters. This scoring system has now been well validated in diverse clinical settings. The theoretical cornerstone of DIC management is the specific and vigorous treatment of the underlying conditions, and DIC should be simultaneously managed to improve patient outcomes. The ISTH guidance for the treatment of DIC recommends treatment strategies that are based on current evidence. In this Primer, we provide an updated overview of the pathophysiology, diagnosis and management of DIC and discuss the future directions of basic and clinical research in this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lasch, H. G., Heene, D. L., Huth, K. & Sandritter, W. Pathophysiology, clinical manifestations and therapy of consumption-coagulopathy (“Verbrauchskoagulopathie”). Am. J. Cardiol. 20, 381–391 (1967).
Spero, J. A., Lewis, J. H. & Hasiba, U. Disseminated intravascular coagulation. Findings in 346 patients. Thromb. Haemost. 43, 28–33 (1980).
Taylor, F. B. Jr et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 86, 1327–1330 (2001).
Wada, H. et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J. Thromb. Haemost. 11, 761–767 (2013). Together with reference 3, these were important landmarks in standardizing and harmonizing the diagnosis of DIC.
[No authors listed.] American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20, 864–874 (1992).
Gando, S., Kameue, T., Nanzaki, S. & Nakanishi, Y. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb. Haemost. 75, 224–228 (1996). This paper links DIC to the systemic inflammatory state.
Bakhtiari, K., Meijers, J. C. M., de Jonge, E. & Levi, M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit. Care Med. 32, 2416–2421 (2004).
Gando, S. et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: Results of a multicenter, prospective survey. Crit. Care Med. 36, 145–150 (2008).
Matsuda, T. et al. in Annual Report of the Research Committee on DIC 1992. (ed. Matsuda, T. ) 17–30 (Ministry of Health and Welfare of Japan, 1993).
Nakagawa, M. in Annual Report of the Research Committee on DIC 1998. (ed. Nakagawa, M. ) 57–64 (Ministry of Health and Welfare of Japan, 1999).
Murata, A., Okamoto, K., Mayumi, T., Muramatsu, K. & Matsuda, S. The recent time trend of outcomes of disseminated intravascular coagulation in Japan: an observational study based on a national administrative database. J. Thromb. Thromblysis 38, 364–371 (2014).
Cartin-Ceba, R. et al. Epidemiology of critical care syndromes, organ failures, and life-support interventions in a suburban US community. Chest 140, 1447–1445 (2011).
Singh, B. et al. Trends in the incidence and outcomes of disseminated intravascular coagulation in critically ill patients (2004–2010): a population-based study. Chest 143, 1235–1242 (2013).
Rangel-Frausto, M. S. et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273, 117–123 (1995). A clinical study that elucidated the interaction between inflammation and coagulation.
Ogura, H. et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J. Infect. Chemother. 20, 157–162 (2014).
Dhainaut, J. F. et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J. Thromb. Haemost. 2, 1924–1933 (2004).
Kienast, J. et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemostasis 4, 90–97 (2006).
Gando, S. et al. A multicenter, prospective validation study of the Japanese association for acute medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit. Care 17, R111 (2013).
Sawamura, A. et al. Application of the Japanese Association for Acute Medicine disseminated intravascular coagulation diagnostic criteria for patients at an early phase of trauma. Thromb. Res. 124, 706–710 (2009).
Oshiro, A. et al. Hemostasis during the early stage of trauma: comparison with disseminated intravascular coagulation. Crit. Care 18, R61 (2014).
Rattray, D., O'Connell, C. M. & Baskett, T. F. Acute disseminated intravascular coagulation in obstetrics: a tertiary center population review (1980 to 2009). J. Obstet. Gynaecol. Can. 34, 341–347 (2012).
Esmon, C. T., Xu, J. & Lupu, F. Innate immunity and coagulation. J. Thromb. Haemost. 9 (Suppl. 1), 182–188 (2011).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Levi, M., van der Poll, T., ten Cate, H. & van Deventer, S. J. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Invest. 27, 3–9 (1997).
Levi, M., van der Poll, T. & Buller, H. R. The bidirectional relationshiop between coagulation and inflammation. Circulation 109, 2698–2704 (2004).
Aird, W. C. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit. Care Med. 29, S28–S34 (2001).
Falanga, A., Schieppati, F. & Russo, D. Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Semin. Thromb. Hemost. 41, 756–764 (2015).
Levi, M. Pathogenesis and management of peripartum coagulopathic calamities (disseminated intravascular coagulation and amniotic fluid embolism). Thromb. Res. 131, S32–S34 (2013).
Franco, R. F. et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood 96, 554–559 (2000).
Osterud, B. & Flaegstad, T. Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb. Haemost. 49, 5–7 (1983).
Taylor, F. B. Jr et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ. Shock 33, 127–134 (1991).
Levi, M. et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J. Clin. Invest. 93, 114–120 (1994).
Gando, S. Hemostasis and thrombosis in trauma patients. Semin. Thromb. Hemost. 41, 26–34 (2015).
Falanga, A., Marchetti, M. & Vignoli, A. Coagulation and cancer: biological and clinical aspects. J. Thromb. Haemost. 11, 223–233 (2013).
Delabranche, X. et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 39, 1695–1703 (2013).
Levi, M. & van der Poll, T. Inflammation and coagulation. Crit. Care Med. 38, S26–S34 (2010).
Giesen, P. L. et al. Blood-borne tissue factor: another view of thrombosis. Proc. Natl Acad. Sci. USA 96, 2311–2315 (1999).
Osterud, B., Rao, L. V. & Olsen, J. O. Induction of tissue factor expression in whole blood — lack of evidence for the presence of tissue factor expression on granulocytes. Thromb. Haemost. 83, 861–867 (2000).
Rauch, U. et al. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 96, 170–175 (2000).
Zimmerman, G. A., McIntyre, T. M., Prescott, S. M. & Stafforini, D. M. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 30, S294–S301 (2002).
Versteeg, H. H., Heemskerk, J. W., Levi, M. & Reitsma, P. H. New fundamentals in hemostasis. Physiol. Rev. 93, 327–358 (2013). A comprehensive paper providing new insights in coagulation physiology and pathology.
Shebuski, R. J. & Kilgore, K. S. Role of inflammatory mediators in thrombogenesis. J. Pharmacol. Exp. Ther. 300, 729–735 (2002).
Eerenberg, E. S. & Levi, M. The potential therapeutic benefit of targeting ADAMTS13 activity. Semin. Thromb. Hemost. 40, 28–33 (2014).
Bockmeyer, C. L. et al. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 93, 137–140 (2008).
Booth, K. K., Terrell, D. R., Vesely, S. K. & George, J. N. Systemic infections mimicking thrombotic thrombocytopenic purpura. Am. J. Hematol. 86, 743–751 (2011).
Kremer Hovinga, J. A. et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J. Thromb. Haemost. 5, 2284–2290 (2007).
Bartlett, A. H., Hayashida, K. & Park, P. W. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol. Cells 24, 153–166 (2007).
Esmon, C. T. The regulation of natural anticoagulant pathways. Science 235, 1348–1352 (1987).
Griffin, J. H., Fernandez, J. A., Gale, A. J. & Mosnier, L. O. Activated protein C. J. Thromb. Haemost. 5 (Suppl. 1), 73–80 (2007).
Esmon, C. T. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost 32 (Suppl. 1), 49–60 (2006).
Fijnvandraat, K. et al. Coagulation activation and tissue necrosis in meningococcal septic shock: severely reduced protein C levels predict a high mortality. Thromb. Haemost. 73, 15–20 (1995).
Taylor, F. B. Jr et al. Role of free protein S and C4b binding protein in regulating the coagulant response to Escherichia coli. Blood 86, 2642–2652 (1995).
Taylor, F. B. et al. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood 97, 1685–1688 (2001).
Levi, M. & van der Poll, T. Coagulation in patients with severe sepsis. Semin. Thromb. Hemost. 41, 9–15 (2015).
Sandset, P. M., Warn-Cramer, B. J., Rao, L. V., Maki, S. L. & Rapaport, S. I. Depletion of extrinsic pathway inhibitor (EPI) sensitizes rabbits to disseminated intravascular coagulation induced with tissue factor: evidence supporting a physiologic role for EPI as a natural anticoagulant. Proc. Natl Acad. Sci. USA 88, 708–712 (1991).
Creasey, A. A. et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J. Clin. Invest. 91, 2850–2856 (1993).
de Jonge, E. et al. Tissue factor pathway inhibitor (TFPI) dose-dependently inhibits coagulation activtion without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood 95, 1124–1129 (2000).
Levi, M. & van der Poll, T. A short contemporary history of disseminated intravascular coagulation. Semin. Thromb. Hemost. 40, 874–880 (2014).
Biemond, B. J. et al. Plasminogen activator and plasminogen activator inhibitor I release during experimental endotoxaemia in chimpanzees: effect of interventions in the cytokine and coagulation cascades. Clin. Sci. (Lond.) 88, 587–594 (1995).
Hack, C. E. Fibrinolysis in disseminated intravascular coagulation. Semin. Thromb. Hemost. 27, 633–638 (2001).
Hinshaw, L. B. et al. Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF alpha). Circ. Shock 30, 279–292 (1990).
Abraham, E. et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factorα in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-α MAb Sepsis Study Group. JAMA 273, 934–941 (1995).
van der Poll, T. et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J. Exp. Med. 179, 1253–1259 (1994).
Stouthard, J. M. et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb. Haemost. 76, 738–742 (1996).
Boermeester, M. A. et al. Interleukin-1 blockade attenuates mediator release and dysregulation of the hemostatic mechanism during human sepsis. Arch. Surg. 130, 739–748 (1995).
Coughlin, S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000).
Kannemeier, C. et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc. Natl Acad. Sci. USA 104, 6388–6393 (2007).
Semeraro, F. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118, 1952–1961 (2011).
Fuchs, T. A., Brill, A. & Wagner, D. D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 32, 1777–1783 (2012).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). This study highlighted the importance of innate immune activation and its coupling to DIC. The study described important pathophysiological roles of NETs.
Brinkmann, V. & Zychlinsky, A. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773–783 (2012).
von Bruhl, M. L. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 (2012).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA 107, 15880–15885 (2010).
Iba, T., Miki, T., Hashiguchi, N., Tabe, Y. & Nagaoka, I. Combination of antithrombin and recombinant thrombomodulin modulates neutrophil cell-death and decreases circulating DAMPs levels in endotoxemic rats. Thromb. Res. 134, 169–173 (2014).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7, e32366 (2012).
Kaneider, N. C., Forster, E., Mosheimer, B., Sturn, D. H. & Wiedermann, C. J. Syndecan-4-dependent signaling in the inhibition of endotoxin-induced endothelial adherence of neutrophils by antithrombin. Thromb. Haemost. 90, 1150–1157 (2003).
Esmon, C. T. New mechanisms for vascular control of inflammation mediated by natural anticoagulant proteins. J. Exp. Med. 196, 561–564 (2002).
Yuksel, M., Okajima, K., Uchiba, M., Horiuchi, S. & Okabe, H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb. Haemost. 88, 267–273 (2002).
Murakami, K. et al. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood 87, 642–647 (1996).
Taylor, F. B. Jr et al. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood 95, 1680–1686 (2000).
Levi, M. et al. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein C deficient mice. Blood 101, 4823–4827 (2003).
Toh, C. H. & Hoots, W. K. & SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J. Thromb. Haemost. 5, 604–606 (2007).
Sivula, M., Tallgren, M. & Pettila, V. Modified score for disseminated intravascular coagulation in the critically ill. Intensive Care Med. 31, 1209–1214 (2005).
Cauchie, P. et al. Diagnosis and prognosis of overt disseminated intravascular coagulation in a general hospital — meaning of the ISTH score system, fibrin monomers, and lipoprotein–C-reactive protein complex formation. Am. J. Hematol. 81, 414–419 (2006).
Venugopal, A. Disseminated intravascular coagulation. Indian J. Anaesth. 58, 603–608 (2014).
Toh, C. H. & Dennis, M. Disseminated intravascular coagulation: old disease, new hope. BMJ 327, 974–977 (2003).
Toh, C. H. & Alhamdi, Y. Current consideration and management of disseminated intravascular coagulation. Hematology Am. Soc. Hematol. Educ. Program 2013, 286–291 (2013).
Narayanan, S. Multifunctional roles of thrombin. Ann. Clin. Lab. Sci. 29, 275–280 (1999).
Kjalke, M. et al. Active site-inactivated factors VIIa, Xa, and IXa inhibit individual steps in a cell-based model of tissue factor-initiated coagulation. Thromb. Haemost. 80, 578–584 (1998).
Rezaie, A. R. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr. Med. Chem. 17, 2059–2069 (2010).
Levin, E. G., Marzec, U., Anderson, J. & Harker, L. A. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J. Clin. Invest. 74, 1988–1995 (1984).
Gelehrter, T. D. & Sznycer-Laszuk, R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J. Clin. Invest. 77, 165–169 (1986).
Wang, W., Boffa, M. B., Bajzar, L., Walker, J. B. & Nesheim, M. E. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 273, 27176–27181 (1998).
Satta, N. et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J. Immunol. 153, 3245–3255 (1994).
Moxon, C. A. et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 13, 1653–1664 (2015).
Moxon, C. A. et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122, 842–851 (2013).
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505 (2013).
Mosnier, L. O., Zlokovic, B. V. & Griffin, J. H. The cytoprotective protein C pathway. Blood 109, 3161–3172 (2007).
Gando, S., Wada, H., Thachil, J. & Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis (ISTH). Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS). J. Thromb. Haemost. 11, 826–835 (2013).
Gando, S., Sawamura, A. & Hayakawa, M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann. Surg. 254, 10–19 (2011).
Geerts, W. H., Code, K. I., Jay, R. M., Chen, E. & Szalai, J. P. A prospective study of venous thromboembolism after major trauma. N. Engl. J. Med. 331, 1601–1606 (1994).
Osterud, B. & Bjorklid, E. The production and availability of tissue thromboplastin in cellular populations of whole blood exposed to various concentrations of endotoxin. An assay for detection of endotoxin. Scand. J. Haematol. 29, 175–184 (1982).
Hellum, M. et al. Microparticle-associated tissue factor activity correlates with plasma levels of bacterial lipopolysaccharides in meningococcal septic shock. Thromb. Res. 133, 507–514 (2014).
Muller, F. et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139, 1143–1156 (2009).
Nesheim, M. E., Tracy, R. P. & Mann, K. G. “Clotspeed,” a mathematical simulation of the functional properties of prothrombinase. J. Biol. Chem. 259, 1447–1453 (1984).
Giles, A. R., Mann, K. G. & Nesheim, M. E. A combination of factor Xa and phosphatidylcholine-phosphatidylserine vesicles bypasses factor VIII in vivo. Br. J. Haematol. 69, 491–497 (1988).
Barranco-Medina, S., Pozzi, N., Vogt, A. D. & Di Cera, E. Histone H4 promotes prothrombin autoactivation. J. Biol. Chem. 288, 35749–35757 (2013).
Eltzschig, H. K. & Collard, C. D. Vascular ischaemia and reperfusion injury. Br. Med. Bull. 70, 71–86 (2004).
Darbousset, R. et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 124, 2575–2585 (2014).
Abrams, S. T. et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 187, 160–169 (2013).
Alhamdi, Y. et al. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit. Care Med. 43, 2094–2103 (2015).
Xu, J., Zhang, X., Monestier, M., Esmon, N. L. & Esmon, C. T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 187, 2626–2631 (2011).
Xu, J. et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 (2009). This study highlighted the importance of innate immune activation and its coupling to DIC. In addition, the study described important pathophysiological roles for extracellular DNA and histones in sepsis and related organ failure.
Nakahara, M. et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE 8, e75961 (2013).
Alhamdi, Y. et al. Circulating histone concentrations differentially affect the predominance of left or right ventricular dysfunction in critical illness. Crit. Care Med. 44, e278–e288 (2016).
Allam, R. et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 23, 1375–1388 (2012).
Fuchs, T. A., Bhandari, A. A. & Wagner, D. D. Histones induce rapid and profound thrombocytopenia in mice. Blood 118, 3708–3714 (2011).
Longstaff, C. et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 288, 6946–6956 (2013).
Kim, J. E., Lee, N., Gu, J. Y., Yoo, H. J. & Kim, H. K. Circulating levels of DNA–histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb. Res. 135, 1064–1069 (2015).
Levi, M., Toh, C., Thachil, J. & Watson, H. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br. J. Haematol. 145, 24–33 (2009).
Mesters, R. M. et al. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood 88, 881–886 (1996).
Dhainaut, J. F. et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit. Care Med. 33, 341–348 (2005).
Kobayashi, N., Maekawa, T., Takada, M., Tanaka, H. & Gonmori, H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl. Haematol. 49, 265–275 (1983).
Wada, H. et al. Outcome of disseminated intravascular coagulation in relation to the score when treatment was begun. Mie DIC Study Group. Thromb. Haemost. 74, 848–852 (1995).
Dempfle, C. E. & Borggrefe, M. Point of care coagulation tests in critically ill patients. Semin. Thromb. Hemost 34, 445–450 (2008).
Levi, M. & Hunt, B. J. A critical appraisal of point-of-care coagulation testing in critically ill patients. J. Thromb. Haemost. 13, 1960–1967 (2015).
Zuckerman, L., Cohen, E., Vagher, J. P., Woodward, E. & Caprini, J. A. Comparison of thrombelastography with common coagulation tests. Thromb. Haemost. 46, 752–756 (1981).
Fourrier, F. Severe sepsis, coagulation, and fibrinolysis: dead end or one way? Crit. Care Med. 40, 2704–2708 (2012).
Grottke, O. et al. Thrombin generation capacity of prothrombin complex concentrate in an in vitro dilutional model. PLoS ONE 8, e64100 (2013).
Grundman, C. et al. Prothrombin overload causes thromboembolic complications in prothrombin complex concentrates: in vitro and in vivo evidence. Thromb. Haemost. 94, 1338–1339 (2005).
Grottke, O. et al. Increasing concentrations of prothrombin complex concentrate induce disseminated intravascular coagulation in a pig model of coagulopathy with blunt liver injury. Blood 118, 1943–1951 (2011).
Schochl, H., Voelckel, W., Maegele, M., Kirchmair, L. & Schlimp, C. J. Endogenous thrombin potential following hemostatic therapy with 4-factor prothrombin complex concentrate: a 7-day observational study of trauma patients. Crit. Care 18, R147 (2014).
Simpson, E. et al. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst. Rev. 3, CD005011 (2012).
Jaimes, F. et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (the HETRASE study). Crit. Care Med. 37, 1185–1196 (2009).
Sakuragawa, N., Hasegawa, H., Maki, M., Nakagawa, M. & Nakashima, M. Clinical evaluation of low-molecular-weight heparin (FR-860) on disseminated intravascular coagulation (DIC) — a multicenter co-operative double-blind trial in comparison with heparin. Thromb. Res. 72, 475–500 (1993).
Samama, M. M. et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N. Engl. J. Med. 341, 793–800 (1999).
Hoffman, M. & Monroe, D. M. 3rd. A cell-based model of hemostasis. Thromb. Haemost. 85, 958–965 (2001).
Levi, M. & van der Poll, T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin. Thromb. Hemost. 34, 459–468 (2008).
Warren, B. L. et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286, 1869–1878 (2001).
Abraham, E. et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 290, 238–247 (2003).
Ranieri, V. M. et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 366, 2055–2064 (2012).
Aoki, N. et al. A comparative double-blind randomized trial of activated protein C and unfractionated heparin in the treatment of disseminated intravascular coagulation. Int. J. Hematol. 75, 540–547 (2002).
Afshari, A., Wetterslev, J., Brok, J. & Moller, A. M. Antithrombin III for critically ill patients. Cochrane Database Syst. Rev. 3, CD005370 (2008).
Levi, M., de Jonge, E., van der Poll, T. & ten Cate, H. Disseminated intravascular coagulation. Thromb. Haemost. 82, 695–705 (1999).
Wiedermann, C. J. & Kaneider, N. C. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul. Fibrinolysis 17, 521–526 (2006).
Gando, S. et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit. Care 17, R297 (2013).
Iba, T., Saitoh, D., Wada, H. & Asakura, H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a secondary survey. Crit. Care 18, 497 (2014).
Tagami, T., Matsui, H., Horiguchi, H., Fushimi, K. & Yasunaga, H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J. Thromb. Haemost. 12, 1470–1479 (2014).
Tagami T. Matsui H. Fushimi K. & Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb. Haemost. 114, 537–545 (2015).
Saito, H. et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a Phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 5, 31–41 (2007). This was the first study that showed efficacy and safety of rhTM.
Tagami, T., Matsui, H., Horiguchi, H., Fushimi, K. & Yasunaga, H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J. Thromb. Haemost. 13, 31–40 (2015).
Yamakawa, K. et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit. Care 15, R123 (2011).
Yamakawa, K. et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 39, 644–652 (2013).
Yamakawa, K. et al. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J. Thromb. Haemost. 13, 508–519 (2015).
Yoshimura, J. et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit. Care 19, 78 (2015). The results reported in this paper indirectly support the concept that the target of anticoagulant factor concentrates is not severe sepsis, but severe sepsis with established DIC.
Vincent, J. L. et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit. Care Med. 41, 2069–2079 (2013).
Marder, V. J., Feinstein, D. I., Colman, R. W. & Levi, M. in Hemostasis and Thrombosis. Basic Principles and Clinical Practice 5th edn (eds Colman, R. W., Marder, V. J., Clowes, A. W., George, J. N. & Goldhaber, S. Z. ) 1571–1600 (Lippincott Williams & Wilkins, 2006).
Asakura, H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J. Intensive Care 2, 20 (2014).
Menell, J. S. et al. Annexin II and bleeding in acute promyelocytic leukemia. N. Engl. J. Med. 340, 994–1004 (1999).
Avvisati, G., ten Cate, J. W., Buller, H. R. & Mandelli, F. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet 2, 122–124 (1989).
de la Serna, J. et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood 111, 3395–3402 (2008).
Brown, J. E. et al. All-trans retinoic acid (ATRA) and tranexamic acid: a potentially fatal combination in acute promyelocytic leukaemia. Br. J. Haematol. 110, 1010–1012 (2000).
Arbuthnot, C. & Wilde, J. T. Haemostatic problems in acute promyelocytic leukaemia. Blood Rev. 20, 289–297 (2006).
Lowenstein, C. J., Morrell, C. N. & Yamakuchi, M. Regulation of Weibel–Palade body exocytosis. Trends Cardiovasc. Med. 15, 302–308 (2005).
Suffredini, A. F., Harpel, P. C. & Parrillo, J. E. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N. Engl. J. Med. 320, 1165–1172 (1989).
Shakur, H. et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 376, 23–32 (2010).
Roberts, I. et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 377, 1096–1101, 1101.e1–1101.e2 (2011). Together with reference 167, these two consecutive studies nicely demonstrated that tranexamic acid given within 3 hours from injury can safely reduce the risk of death in patients with bleeding trauma.
Roberts, I. & Prieto-Merino, D. Applying results from clinical trials: tranexamic acid in trauma patients. J. Intensive Care 2, 56 (2014).
Fourrier, F. et al. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 104, 882–888 (1993).
Sawamura, A. et al. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb. Res. 124, 608–613 (2009).
Levi, M., van der Poll, T. & Schultz, M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin. Immunopathol. 34, 167–179 (2012).
Rosenberg, R. D. & Aird, W. C. Vascular-bed-specific hemostasis and hypercoagulable states. N. Engl. J. Med. 340, 1555–1564 (1999).
Texereau, J., Pene, F., Chiche, J. D., Rousseau, C. & Mira, J. P. Importance of hemostatic gene polymorphisms for susceptibility to and outcome of severe sepsis. Crit. Care Med. 32, S313–S319 (2004).
Schouten, M., van 't Veer, C., van der Poll, T. & Levi, M. Effect of the factor V Leiden mutation on the incidence and outcome of severe infection and sepsis. Neth. J. Med. 70, 306–310 (2012).
Hermans, P. W. et al. 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet 354, 556–560 (1999).
US National Library of Science. Phase 3 safety and efficacy study of ART-123 in subjects with severe sepsis and coagulopathy. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01598831 (2012).
Acknowledgements
C.-H.T. has received funding from the US National Institute of Health Research. The authors thank Y. Alhamdi (University of Liverpool, UK) for assistance in preparing the manuscript.
Author information
Authors and Affiliations
Contributions
Introduction (S.G.); Epidemiology (S.G.); Mechanisms/pathophysiology (C.-H.T. and M.L.); Diagnosis, screening and prevention (C.-H.T.); Management (S.G.); Quality of life (S.G.); Outlook (M.L.); Overview of Primer (S.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Gando, S., Levi, M. & Toh, CH. Disseminated intravascular coagulation. Nat Rev Dis Primers 2, 16037 (2016). https://doi.org/10.1038/nrdp.2016.37
Published:
DOI: https://doi.org/10.1038/nrdp.2016.37
This article is cited by
-
How to manage coagulopathies in critically ill patients
Intensive Care Medicine (2023)
-
The Effect of Extracellular Vesicles on Thrombosis
Journal of Cardiovascular Translational Research (2023)
-
Association of CDSS score and 60-day mortality in Chinese patients with non-APL acute myeloid leukemia: a retrospective cohort study
Journal of Thrombosis and Thrombolysis (2023)
-
Sepsis-related coagulopathy treatment based on the disseminated intravascular coagulation diagnostic criteria: a post-hoc analysis of a prospective multicenter observational study
Journal of Intensive Care (2023)
-
An interpretable DIC risk prediction model based on convolutional neural networks with time series data
BMC Bioinformatics (2022)