Key Points
-
Disorders that alter bone function, such as osteoporosis, occur with increasing frequency in the elderly and represent a major public health problem. For example, the economic burden of osteoporosis alone is estimated to be US $14 billion per year in the United States.
-
Understanding normal bone biology is crucial to treating skeletal disease, and identification of mutated genes that cause inherited bone disorders, as well as the use of transgenic and knockout mouse models, has greatly advanced this understanding and has provided potential targets for drug development.
-
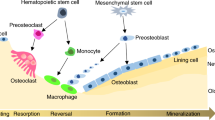
The main cell that contributes to bone formation is the osteoblast, which produces and secretes the structural components of the bone matrix (notably type I collagen) and releases minerals, growth factors and enzymes into it.
-
The cells in bone that are specialized to resorb mineralized matrix are multinucleated osteoclasts, which secrete acid to dissolve matrix minerals and proteases to degrade exposed matrix proteins, through a specialized apical cell-membrane surface that is known as the ruffled border.
-
So far, the most successful therapies for bone disease have targeted the bone-resorbing osteoclastic system. These anti-resorbing agents include bisphosphonates, oestrogens, selective oestrogen-receptor modulators (SERMs) and calcitonin.
-
Future development of anti-resorptive agents might target osteoclast formation, activity and/or cell death at multiple loci being discovered by genetic and genomic approaches.
-
The paucity of available anabolic agents for skeletal disorders makes this a particularly fruitful area for drug development. These agents should be of major benefit in systemic diseases, such as osteoporosis, when significant bone loss has already occurred, or for secondary prevention of fractures, but might also be beneficial in local disorders, such as delayed fracture healing, to accelerate bone formation.
Abstract
Bone turnover, in which cells of the osteoclast lineage resorb bone and cells of the osteoblast lineage deposit bone, normally occurs in a highly regulated manner throughout life. Perturbations to these processes underlie skeletal disorders, such as osteoporosis, which are common, chronic and disabling, and increase with age. On the basis of empirical observations or on understanding of the endocrinology of the skeleton, excellent bone-resorption inhibitors, but few anabolic agents, have been developed as therapeutics for skeletal disorders. However, powerful new genomic and genetic tools are uncovering new loci that regulate the activity of both osteoclasts and osteoblasts, and these hold great promise for future drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cummings, S. R. & Melton, L. J. Epidemiology and outcomes of osteoporotic fractures. Lancet 359, 1761–1767 (2002).
Doherty, D. A., Sanders, K. M., Kotowicz, M. A. & Prince, R. L. Lifetime and five-year age-specific risks of first and subsequent osteoporotic fractures in postmenopausal women. Osteoporosis Int. 12, 16–23 (2001).
Ray, N. F., Chan, J. K., Thamer, M. & Melton, L. J. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J. Bone Miner. Res. 12, 24–35 (1997).
Burr, D. B. The contribution of the organic matrix to bone's material properties. Bone 31, 8–11 (2002).
Kanis, J. A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359, 1929–1936 (2002).
Hunziker, E. B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10, 432–463 (2002).
Klareskog, L., Lorentzen, J., Padyukov, L. & Alfredsson, L. Genes and environment in arthritis: can RA be prevented? Arthritis Res. 4 (Suppl. 3), S31–S36 (2002).
Goltzman, D., Karaplis, A. C., Kremer, R. & Rabbani, S. A. Molecular basis of the spectrum of skeletal complications of neoplasia. Cancer 88 (Suppl. 12), 2903–2908 (2000).
Gemmell, E., Yamazaki, K. & Seymour, G. J. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit. Rev. Oral Biol. Med. 13, 17–34 (2002).
Reddy, S. V., Kurihara, N., Menaa, C. & Roodman, G. D. Paget's disease of bone: a disease of the osteoclast. Rev. Endocr. Metab. Disord. 2, 195–201 (2001).
Econs, M. J. New insights into the pathogenesis of inherited phosphate wasting disorders. Bone 25, 131–135 (1999).
White, K. E. et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nature Genet. 26, 345–348 (2000).The initial description of the discovery of FGF23 in a syndrome of hypophosphataemic rickets.
Mawer, E. B. & Davies, M. Vitamin D nutrition and bone disease in adults. Rev. Endocr. Metab. Disord. 2, 175–186 (2001).
Aubin, J. E. Regulation of osteoblast formation and function. Rev. Endocr. Metab. Disord. 2, 81–94 (2001).
Komori, T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002).
Sabatakos, G. et al. Overexpression of ΔFOSB transcription factor(s) increases bone formation and inhibits adipogenesis. Nature Med. 6, 985–990 (2000).
Jochum, W. et al. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nature Med. 6, 985–990 (2000).
Rouleau, M. F., Mitchell, J. & Goltzman, D. In vivo distribution of parathyroid hormone receptor in bone: evidence that a predominant osseous target cell is not the mature osteoblast. Endocrinology 123, 187–191 (1988).
Suda, T., Kobayashi, K., Jimi, E., Udagawa, N. & Takahashi, N. The molecular basis of osteoclast differentiation and activation. Novartis Found. Symp. 232, 235–247 (2001).
Watanuki, M. et al. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 17, 1015–1025 (2002).
Miao, D., He, B., Karaplis, A. C. & Goltzman, D. Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest. 109, 1173–1182 (2002).The first report of the physiological role of PTH as an anabolic agent in fetal bone.
Amizuka, N. et al. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 175, 166–176 (1996).
Miao, D. et al. Parathyroid hormone-related peptide stimulates osteogenic cell proliferation through protein kinase C activation of the Ras/mitogen-activated protein kinase signaling pathway. J. Biol. Chem. 276, 32204–32213 (2001).
Carpio, L., Gladu, J., Goltzman, D. & Rabbani, S. A. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am. J. Physiol. Endocrinol. Metab. 281, E489–E499 (2001).
Jilka, R. L. et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 104, 439–446 (1999).A description of the inhibition of apoptosis as an important mechanism for the anabolic effect of PTH.
Rodan, G. A. & Martin, T. J. Therapeutic approaches to bone diseases. Science 289, 1508–1514 (2000).
Dempster, D. W. et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J. Bone Miner. Res. 16, 1846–1853 (2001).
Canalis, E. Novel treatments for osteoporosis. J. Clin. Invest. 106, 177–179 (2000).
Gong, Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001).A ground-breaking report of the involvement of LRP5 in bone formation.
Little, C. D. et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70, 11–19 (2002).
Boyden, L. M. et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346, 1513–1521 (2002).
Kato, M. et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157, 303–314 (2002).
Mao, B. et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signaling. Nature 417, 664–667 (2002).
Hunter, I., McGregor, D. & Robins, S. P. Caspase-dependent cleavage of cadherins and catenins during osteoblast apoptosis. J. Bone Miner. Res. 16, 466–477 (2001).
Marie, P. J. Role of N-cadherin in bone formation. J. Cell. Physiol. 190, 297–305 (2002).
Lecanda, F. et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 151, 931–944 (2000).
Tanaka, Y. et al. H-Ras/mitogen-activated protein kinase pathway inhibits integrin-mediated adhesion and induces apoptosis in osteoblasts. J. Biol. Chem. 277, 21446–21452 (2002).
Bianco, P., Riminucci, M., Gronthos, S. & Robey, P. G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19, 180–192 (2001).
Nuttall, M. E. & Gimble, J. M. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 27, 177–184 (2000).
Capdevila, J. & Belmonte, J. C. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 17, 87–132 (2001).
Garrett, I. R. & Mundy, G. R. The role of statins as potential targets for bone formation. Arthritis Res. 4, 237–240 (2002).
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).An excellent review of the molecular regulation of osteoclast function.
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-Src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Sanjay, A., et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, αvβ3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152, 181–196 (2001).
Hu, P. Y. et al. A splice junction mutation in intron 2 of the carbonic anhydrase II gene of osteopetrosis patients from Arabic countries. Hum. Mutat. 1, 288–292 (1992).
Teti, A. et al. Cytoplasmic pH regulation and chloride/bicarbonate exchange in avian osteoclasts. J. Clin. Invest. 83, 227–233 (1989).
Blair, H. C., Teitelbaum, S. L., Ghiselli, R. & Gluck, S. Ostoeclastic bone resorption by a polarized vacuolar proton pump. Science 245, 855–857 (1989).
Stroup, G. B. et al. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J. Bone Miner. Res. 16, 1747–1749 (2001).
Tondravi, M. M. et al. Osteopetrosis in mice lacking hematopoietic transcription factor PU.1. Nature 386, 81–84 (1997).
Grigoriadis, A. E. et al. c-Fos: a key regulator of osteoclast–macrophage lineage determination and bone remodeling. Science 266, 443–448 (1994).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nature Med. 3, 1285–1289 (1997).
Hodgkinson, C. A. et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic helix–loop–helix zipper protein. Cell 74, 395–404 (1993).
Theill, L. E., Boyle, W. J. & Penninger, J. M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20, 795–823 (2002).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).An initial description of the role of OPG in the regulation of bone density.
Takayanagi, H. et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000).
Delmas, P. D. Treatment of postmenopausal osteoporosis. Lancet 359, 2018–2026 (2002).An excellent review of the current therapy for postmenopausal osteoporosis.
Russell, R. G. et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J. Bone Miner. Res. 14 (Suppl. 2), 53–65 (1999).
Lehenkari, P. P. et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 61, 1255–1262 (2002).
Lindsay, R, Hart, D. M. & Fogelman, I. Bone mass after withdrawal of oestrogen replacement. Lancet 1, 729 (1981).
Manolagas, S. C., Kousteni, S. & Jilka, R. L. Sex steroids and bone. Recent Prog. Horm. Res. 57, 385–409 (2002).
Riggs, B. L., Khosla, S. & Melton, L. J. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 23, 279–302 (2002).References 61 and 62 are both comprehensive reviews of the effect of sex steroids on bone.
Lufkin, E. G., Wong, M. & Deal, C. The role of selective estrogen receptor modulators in the prevention and treatment of osteoporosis. Rheum. Dis. Clin. North Am. 27, 163–185 (2001).
Martin, T. J. Calcitonin, an update. Bone 24 (Suppl. 5), 63S–65S (1999).
Nguyen, T. V. & Eisman, J. A. Genetics of fracture: challenges and opportunities. J. Bone Miner. Res. 15, 1243–1252 (2000).
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2, 389–406 (2002).
Kim, H. J., Rice, D. P., Kettunen, P. J. & Thesleff, I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 125, 1241–1251 (1998).
Suda, N. et al. Parathyroid hormone-related protein is required for normal intramembranous bone development. J. Bone Miner. Res. 16, 2182–2191 (2001).
Zhang, X. et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Invest. 109, 1405–1415 (2002).
Bi, W. et al. Sox9 is required for cartilage formation. Nature Genet. 22, 85–89 (1999).
Smits, P. et al. The transcription factors l-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1, 277–290 (2001).
Panda, D. K. et al. The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells. J. Biol. Chem. 276, 41229–41236 (2001).
Amizuka, N., Warshawsky, H., Henderson, J. E., Goltzman, D. & Karaplis, A. C. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J. Cell Biol. 126, 1611–1623 (1994).A demonstration of the crucial role of PTHrP in endochondral bone formation.
Chung, U. I., Schipani, E., McMahon, A. P. & Kronenberg, H. M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Invest. 107, 295–304 (2001).
Ornitz, D. M. & Marie, P. J. FGF signaling pathways in endochondral and intramembraneous bone development and human genetic disease. Genes Dev. 16, 1446–1465 (2002).
Zelzer, E. et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129, 1893–1904 (2002).
St-Jacques, B., Hammerschmidt, M. & McMahon, A. P. Indian hedgehog signalling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072–2086 (1999).
Takeda, S. & Karsenty, G. Central control of bone formation. J. Bone Miner. Metab. 19, 195–198 (2001).
Panda, D. K. et al. Targeted ablation of the 25-hydroxyvitamin D1 α-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl Acad. Sci. USA 98, 7498–7503 (2001).
Amling, M. et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140, 4982–4987 (1999).
Mosekilde, L. Mechanisms of age-related bone loss. Novartis Found. Symp. 235, 150–166 (2001).
Norman, T. L. & Wang, Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone 20, 375–379 (1997).
Gazit, D., Zilberman, Y., Ebner, R. & Kahn, A. Bone loss (osteopenia) in old male mice results from diminished activity and availability of TGF-β. J. Cell. Biochem. 70, 478–488 (1998).
Aerssens, J., Boonen, S., Joly, J. & Dequeker, J. Variations in trabecular bone composition with anatomical site and age: potential implications for bone quality assessment. J. Endocrinol. 155, 411–421 (1997).
Seeman, E. Pathogenesis of bone fragility in women and men. Lancet 359, 1841–1850 (2002).
Delmas, P. D. et al. The use of placebo-controlled and non-inferiority trials for the evaluation of new drugs in the treatment of postmenopausal osteoporosis. Osteoporosis Int. 13, 1–5 (2002).A description of the ethical dilemma in the use of placebo controls for treatment of osteoporosis.
The Writing Group for the PEPI. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA 276, 1389–1396 (1996).
Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).A landmark report on the effects of combinations of oestrogen and progesterone on bone and on other organs in postmenopausal women.
Ettinger, B. et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282, 637–645 (1999).
Barrett-Connor, E. et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 287, 847–857 (2002).
Watts, N. B. Treatment of osteoporosis with bisphosphonates. Rheum. Dis. Clin. North. Am. 27, 197–214 (2001).
Hosking, D. et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N. Engl. J. Med. 338, 485–492 (1998).
Mortensen, L. et al. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J. Clin. Endocrinol. Metab. 83, 396–402 (1998).
Schnitzer, T. et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging (Milano) 12, 1–12 (2000).
Cummings, S. R. et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280, 2077–2082 (1998).
Harris, S. T. et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282, 1344–1352 (1999).
Black, D. M. et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348, 1535–1541 (1996).
McClung, M. R. et al. Effect of risedronate on the risk of hip fracture in elderly women. N. Engl. J. Med. 344, 333–340 (2001).
Ringe, J. D., Orwall, E., Daifotis, A. & Lombardi, A. Treatment of male osteoporosis: recent advances with alendronate. Osteoporosis Int. 13, 195–199 (2002).
Saag, K. G. et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N. Engl. J. Med. 339, 292–299 (1998).
Reid, I. R. et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N. Engl. J. Med. 346, 653–661 (2002).
Siris, E. S. Goals of treatment for Paget's disease of bone. J. Bone Miner. Res. 14 (Suppl. 2), 49–52 (1999).
Coleman, R. E. Optimising treatment of bone metastases by Aredia and Zometa. Breast Cancer 7, 361–369 (2000).
Glorieux, F. H. et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N. Engl. J. Med. 339, 947–952 (1998).
Lehmann, H. J., Mouritzen, U., Christgau, S., Cloos, P. A. & Christiansen, C. Effect of bisphosphonates on cartilage turnover assessed with a newly developed assay for collagen type II degradation products. Ann. Rheum. Dis. 61, 530–533 (2002).
Chesnut, C. H. et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am. J. Med. 109, 267–276 (2000).
Selye, H. On stimulation of new bone-formation with parathyroid extract and irradiated ergosterol. Endocrinology 16, 547–558 (1932).
Reeve, J. et al. The anabolic effect of low doses of human parathyroid hormone fragment on the skeleton in postmenopausal osteoporosis. Lancet 1, 1035–1038 (1976).
Tam, C. S., Heersche, J. N., Murray, T. M. & Parsons, J. A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110, 506–512 (1982).
Dobnig, H. & Turner, R. T. The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138, 4607–4612 (1997).
Neer, R. M. et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).The first report of the beneficial effect of PTH treatment on fractures in postmenopausal osteoporosis.
Brown, A. J. Therapeutic uses of vitamin D analogues. Am. J. Kidney Dis. 38 (Suppl. 5), S3–S19 (2001).
Dawson-Hughes, B., Harris, S. S., Krall, E. A. & Dallal, G. E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 337, 670–676 (1997).
Chapuy, M. C. et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N. Engl. J. Med. 327, 1637–1642 (1992).
Negro-Vilar, A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J. Clin. Endocrinol. Metab. 84, 3459–3462 (1999).
Lieberman, J. R., Daluiski, A. & Einhorn, T. A. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Joint Surg. Am. 84, A1032–A1044 (2002).
Nemeth, E. F. et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J. Pharmacol. Exp. Ther. 299, 323–331 (2001).
Meunier, P. J. et al. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis — a 2-year randomized placebo controlled trial. J. Clin. Endocrinol. Metab. 87, 2060–2066 (2002).
Reginster, J. Y. Strontium ranelate in osteoporosis. Curr. Pharm. Des. 8, 19079–1916 (2002).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
calcium receptor of the parathyroid glands
Medscape DrugInfo
OMIM
Glossary
- METASTASIS
-
The movement or spreading of cancer cells from one tissue or organ.
- BONE MINERAL DENSITY
-
A two-dimensional (areal) measure of the mineral content of bone in a particular skeletal region, which is performed by a technique called bone densitometry.
- SUBCHONDRAL
-
Beneath the cartilage.
- HYPERTROPHY
-
An increase in the size of a tissue or organ that results from an increase in the size of the cells present.
- MESENCHYME
-
The meshwork of embryonic tissue in the mesoderm (one of the primary embryonic germ layers), from which are formed the connective tissues of the body, as well as bone, cartilage, muscle, blood and lymphatic vessels.
- HAEMATOGENOUS
-
Produced by, or derived from, cells that give rise to blood cells.
- ENDOCHONDRAL
-
Occurring with cartilage.
- CHONDROCYTE
-
A cartilage cell.
- VASOMOTOR
-
Affecting blood-vessel calibre and causing transient, episodic redness of the face and neck (hot flushes).
Rights and permissions
About this article
Cite this article
Goltzman, D. Discoveries, drugs and skeletal disorders. Nat Rev Drug Discov 1, 784–796 (2002). https://doi.org/10.1038/nrd916
Issue Date:
DOI: https://doi.org/10.1038/nrd916
This article is cited by
-
Mesenchymal stem cells: amazing remedies for bone and cartilage defects
Stem Cell Research & Therapy (2020)
-
Effects of Sympathetic Activity on Human Skeletal Homeostasis: Clinical Evidence from Pheochromocytoma
Clinical Reviews in Bone and Mineral Metabolism (2019)
-
Potential damaging mutation in LRP5 from genome sequencing of the first reported chimpanzee with the Chiari malformation
Scientific Reports (2017)
-
Comparison of the alendronate and irradiation with a light-emitting diode (LED) on murine osteoclastogenesis
Lasers in Medical Science (2017)
-
Role of Endoplasmic Reticulum Stress and Unfolded Protein Responses in Health and Diseases
Indian Journal of Clinical Biochemistry (2016)