Key Points
-
Hearing loss is the most common form of sensory impairment in humans and manifests in many forms, ranging from deafness at birth to slow progressive hearing loss during the ageing process.
-
Hearing loss is caused by both genetic and environmental factors.
-
Treatment options for hearing loss are mostly based on medical devices, including hearing aids and cochlear implants; there are no pharmacological therapeutics currently in widespread use.
-
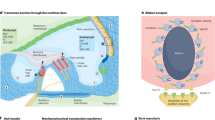
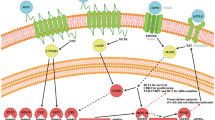
Hair cells in the inner ear are the sensory cells for the detection of sound; hair cells are frequently affected in different forms of hearing loss and they are a common target for therapeutic intervention in various forms of the disease.
-
The study of genes that are affected in patients suffering from hearing loss has identified molecular pathways that are potential therapeutic targets.
-
Hair cells have been generated in vitro from stem cells and hold great potential for drug screens and possibly for regenerative medicine.
-
Gene therapy has successfully been used to treat hearing loss in animal model systems.
-
Different forms of hearing loss, such as noise- and age-related forms, share pathological features, which raises the possibility that similar approaches and pharmacological therapeutics might be useful to treat different forms of the disease.
Abstract
Hearing loss is the most common form of sensory impairment in humans and affects more than 40 million people in the United States alone. No drug-based therapy has been approved by the Food and Drug Administration, and treatment mostly relies on devices such as hearing aids and cochlear implants. Over recent years, more than 100 genetic loci have been linked to hearing loss and many of the affected genes have been identified. This understanding of the genetic pathways that regulate auditory function has revealed new targets for pharmacological treatment of the disease. Moreover, approaches that are based on stem cells and gene therapy, which may have the potential to restore or maintain auditory function, are beginning to emerge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thompson, D. C. et al. Universal newborn hearing screening: summary of evidence. JAMA 286, 2000–2010 (2001).
Lin, F. R., Thorpe, R., Gordon-Salant, S. & Ferrucci, L. Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, 582–590 (2011).
Hong, O., Kerr, M. J., Poling, G. L. & Dhar, S. Understanding and preventing noise-induced hearing loss. Dis. Mon. 59, 110–118 (2013).
Angeli, S., Lin, X. & Liu, X. Z. Genetics of hearing and deafness. Anat. Rec. (Hoboken) 295, 1812–1829 (2012).
Richardson, G. P., de Monvel, J. B. & Petit, C. How the genetics of deafness illuminates auditory physiology. Annu. Rev. Physiol. 73, 311–334 (2011).
Dror, A. A. & Avraham, K. B. Hearing impairment: a panoply of genes and functions. Neuron 68, 293–308 (2010).
Raviv, D., Dror, A. A. & Avraham, K. B. Hearing loss: a common disorder caused by many rare alleles. Ann. NY Acad. Sci. 1214, 168–179 (2010).
Schwander, M., Kachar, B. & Müller, U. The cell biology of hearing. J. Cell Biol. 190, 9–20 (2010).
Mann, Z. F. & Kelley, M. W. Development of tonotopy in the auditory periphery. Hear. Res. 276, 2–15 (2011).
Hudspeth, A. J. How hearing happens. Neuron 19, 947–950 (1997).
Hudspeth, A. J. Making an effort to listen: mechanical amplification in the ear. Neuron 59, 530–545 (2008).
Steel, K. P. in Genes and Common Diseases: Genetics in Modern Medicine ( eds Wright, A. F. & Hastie, N. D. ) 505–515 (Cambridge Univ. Press, 2007).
Friedman, T. B., Schultz, J. M., Ahmed, Z. M., Tsilou, E. T. & Brewer, C. C. Usher syndrome: hearing loss with vision loss. Adv. Otorhinolaryngol 70, 56–65 (2011).
Kemperman, M. H., Hoefsloot, L. H. & Cremers, C. W. Hearing loss and connexin 26. J. R. Soc. Med. 95, 171–177 (2002).
Shin, J. B. et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron 53, 371–386 (2007).
Barnham, K. J., Masters, C. L. & Bush, A. I. Neurodegenerative diseases and oxidative stress. Nature Rev. Drug Discov. 3, 205–214 (2004).
Kikuchi, T., Adams, J. C., Miyabe, Y., So, E. & Kobayashi, T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med. Electron. Microsc. 33, 51–56 (2000).
Dobie, R. A. The burdens of age-related and occupational noise-induced hearing loss in the United States. Ear Hear. 29, 565–577 (2008).
CDC. Severe hearing impairment among military veterans — United States, 2010. MMWR Morb. Mortal. Wkly Rep. 60, 955–958 (2011).
Oishi, N. & Schacht, J. Emerging treatments for noise-induced hearing loss. Expert Opin. Emerg. Drugs 16, 235–245 (2011).
Humes, L. E. & Joellenbeck, L. Noise and Military Service: Implications for Hearing Loss and Tinnitus. (National Academies Press, 2005).
Clark, W. W. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. J. Acoust. Soc. Am. 90, 155–163 (1991).
Kujawa, S. G. & Liberman, M. C. Adding insult to injury: cochlear nerve degeneration after 'temporary' noise-induced hearing loss. J. Neurosci. 29, 14077–14085 (2009).
Maison, S. F., Usubuchi, H. & Liberman, M. C. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J. Neurosci. 33, 5542–5552 (2013).
Furman, A. C., Kujawa, S. G. & Liberman, M. C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 110, 577–586 (2013).
Plack, C. J., Barker, D. & Prendergast, G. Perceptual consequences of 'hidden' hearing loss. Trends Hear. 18, 2331216514550621 (2014).
Sliwinska-Kowalska, M. & Pawelczyk, M. Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutat. Res. 752, 61–65 (2013).
Taleb, M. et al. Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death. Cell Stress Chaperones 14, 427–437 (2009).
Moore, R. D., Smith, C. R. & Lietman, P. S. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J. Infect. Dis. 149, 23–30 (1984).
Lerner, S. A., Schmitt, B. A., Seligsohn, R. & Matz, G. J. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am. J. Med. 80, 98–104 (1986).
Fausti, S. A., Frey, R. H., Henry, J. A., Olson, D. J. & Schaffer, H. I. Early detection of ototoxicity using high-frequency, tone-burst-evoked auditory brainstem responses. J. Am. Acad. Audiol. 3, 397–404 (1992).
Li, H. & Steyger, P. S. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci. Rep. 1, 159 (2011).
Marcotti, W., van Netten, S. M. & Kros, C. J. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 567, 505–521 (2005). This paper describes the mechanisms by which dihydrostreptomycin enters hair cells to cause hair cell death.
Agrawal, R. K. & Sharma, M. R. Structural aspects of mitochondrial translational apparatus. Curr. Opin. Struct. Biol. 22, 797–803 (2012).
Hu, D. N. et al. Genetic aspects of antibiotic induced deafness: mitochondrial inheritance. J. Med. Genet. 28, 79–83 (1991).
Matt, T. et al. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc. Natl Acad. Sci. USA 109, 10984–10989 (2012).
Ding, Y., Leng, J., Fan, F., Xia, B. & Xu, P. The role of mitochondrial DNA mutations in hearing loss. Biochem. Genet. 51, 588–602 (2013).
Langer, T., am Zehnhoff-Dinnesen, A., Radtke, S., Meitert, J. & Zolk, O. Understanding platinum-induced ototoxicity. Trends Pharmacol. Sci. 34, 458–469 (2013).
Ciarimboli, G. et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 176, 1169–1180 (2010).
Riedemann, L. et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenom. J. 8, 23–28 (2008).
Xu, X. et al. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer 77, 438–442 (2012).
Ross, C. J. et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature Genet. 41, 1345–1349 (2009).
Pussegoda, K. et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin. Pharmacol. Ther. 94, 243–251 (2013).
Dalton, D. S. et al. The impact of hearing loss on quality of life in older adults. Gerontologist 43, 661–668 (2003).
Heine, C. & Browning, C. J. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil. 24, 763–773 (2002).
Gates, G. A. & Mills, J. H. Presbycusis. Lancet 366, 1111–1120 (2005).
Yamasoba, T. et al. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear. Res. 303, 30–38 (2013).
Schuknecht, H. F. Presbycusis. Trans. Am. Laryngol. Rhinol. Otol. Soc. 401–418; discussion 419–420 (1955).
Schuknecht, H. F. Further observations on the pathology of presbycusis. Arch. Otolaryngol. 80, 369–382 (1964).
Schuknecht, H. F. & Gacek, M. R. Cochlear pathology in presbycusis. Ann. Otol. Rhinol. Laryngol. 102, 1–16 (1993).
Nelson, E. G. & Hinojosa, R. Age-related histopathologic changes in the human cochlea. Otolaryngol. Head Neck Surg. 133, 817 (2005).
Ohlemiller, K. K. Age-related hearing loss: the status of Schuknecht's typology. Otolaryngol. Head Neck Surg. 12, 439–443 (2004). This paper provides a critical review that highlights the uses and limitations of the definition of the pathology of ARHL using Schuknecht's typology.
Huang, Q. & Tang, J. Age-related hearing loss or presbycusis. Eur. Arch. Otorhinolaryngol 267, 1179–1191 (2010).
Gates, G. A., Couropmitree, N. N. & Myers, R. H. Genetic associations in age-related hearing thresholds. Arch. Otolaryngol. Head Neck Surg. 125, 654–659 (1999).
Karlsson, K. K., Harris, J. R. & Svartengren, M. Description and primary results from an audiometric study of male twins. Ear Hear. 18, 114–120 (1997).
Christensen, K., Frederiksen, H. & Hoffman, H. J. Genetic and environmental influences on self-reported reduced hearing in the old and oldest old. J. Am. Geriatr. Soc. 49, 1512–1517 (2001).
Huyghe, J. R. et al. Genome-wide SNP-based linkage scan identifies a locus on 8q24 for an age-related hearing impairment trait. Am. J. Hum. Genet. 83, 401–407 (2008).
Van Laer, L. et al. A genome-wide association study for age-related hearing impairment in the Saami. Eur. J. Hum. Genet. 18, 685–693 (2010).
Van Laer, L. et al. The grainyhead like 2 gene (GRHL2), alias TFCP2L3, is associated with age-related hearing impairment. Hum. Mol. Genet. 17, 159–169 (2008).
Friedman, R. A. et al. GRM7 variants confer susceptibility to age-related hearing impairment. Hum. Mol. Genet. 18, 785–796 (2009). This paper describes a gene association study that links polymorphisms in GRM7 to ARHL. GRM7 is, so far, the only gene that seems to achieve genome-wide significance in gene association studies for ARHL.
Newman, D. L. et al. GRM7 variants associated with age-related hearing loss based on auditory perception. Hear. Res. 294, 125–132 (2012). This study reinforces the link between polymorphisms in GRM7 and ARHL.
Van Eyken, E. et al. Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J. Med. Genet. 44, 570–578 (2007).
Bared, A. et al. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol. Head Neck Surg. 143, 263–268 (2010).
Unal, M. et al. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope 115, 2238–2241 (2005).
Arnett, J. et al. Autosomal dominant progressive sensorineural hearing loss due to a novel mutation in the KCNQ4 gene. Arch. Otolaryngol. Head Neck Surg. 137, 54–59 (2011).
Uchida, Y., Sugiura, S., Nakashima, T., Ando, F. & Shimokata, H. Endothelin-1 gene polymorphism and hearing impairment in elderly Japanese. Laryngoscope 119, 938–943 (2009).
Sugiura, S., Uchida, Y., Nakashima, T., Ando, F. & Shimokata, H. The association between gene polymorphisms in uncoupling proteins and hearing impairment in Japanese elderly. Acta Otolaryngol. 130, 487–492 (2010).
Seidman, M. D. et al. Association of mitochondrial DNA deletions and cochlear pathology: a molecular biologic tool. Laryngoscope 106, 777–783 (1996).
Bai, U., Seidman, M. D., Hinojosa, R. & Quirk, W. S. Mitochondrial DNA deletions associated with aging and possibly presbycusis: a human archival temporal bone study. Am. J. Otol. 18, 449–453 (1997).
Markaryan, A., Nelson, E. G. & Hinojosa, R. Quantification of the mitochondrial DNA common deletion in presbycusis. Laryngoscope 119, 1184–1189 (2009).
Zheng, Q. Y., Johnson, K. R. & Erway, L. C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 130, 94–107 (1999). In this study, the comprehensive testing of auditory function in inbred mouse strains reveals genetic background-dependent differences in auditory sensitivity and in susceptibility to ARHL.
Johnson, K. R., Zheng, Q. Y. & Noben-Trauth, K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 1091, 79–88 (2006).
Johnson, K. R., Longo-Guess, C., Gagnon, L. H., Yu, H. & Zheng, Q. Y. A locus on distal chromosome 11 (ahl8) and its interaction with Cdh23ahl underlie the early onset, age-related hearing loss of DBA/2J mice. Genomics 92, 219–225 (2008).
Zheng, Q. Y., Ding, D., Yu, H., Salvi, R. J. & Johnson, K. R. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol. Aging 30, 1693–1705 (2009).
Ohmen, J. et al. Genome-wide association study for age-related hearing loss (AHL) in the mouse: a meta-analysis. J. Assoc. Res. Otolaryngol. 15, 335–352 (2014).
Ohlemiller, K. K. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 1277, 70–83 (2009).
Fetoni, A. R., Picciotti, P. M., Paludetti, G. & Troiani, D. Pathogenesis of presbycusis in animal models: a review. Exp. Gerontol. 46, 413–425 (2011).
Mehrparvar, A. H. et al. Conventional audiometry, extended high-frequency audiometry, and DPOAE for early diagnosis of NIHL. Iran. Red Crescent Med. J. 16, e9628 (2014).
Shearer, A. E. & Smith, R. J. Genetics: advances in genetic testing for deafness. Curr. Opin. Pediatr. 24, 679–686 (2012).
Lin, X. et al. Applications of targeted gene capture and next-generation sequencing technologies in studies of human deafness and other genetic disabilities. Hear. Res. 288, 67–76 (2012).
Rubel, E. W., Furrer, S. A. & Stone, J. S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 297, 42–51 (2013).
Hu, Z. & Ulfendahl, M. The potential of stem cells for the restoration of auditory function in humans. Regen. Med. 8, 309–318 (2013).
Geleoc, G. S. & Holt, J. R. Sound strategies for hearing restoration. Science 344, 1241062 (2014).
Kiernan, A. E. et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035 (2005). This study identifies Sox2 as an upstream regulator for the expression of Atoh1 , which is essential for hair cell development. This paper thus provides the first insights into the transcriptional programme that is necessary to specify differentiation of pluripotent cells into hair cells in vitro.
Ahmed, M. et al. Eya1–Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 22, 377–390 (2012). This paper identifies EYA1–SIX1 as an additional component of the transcriptional network that also contains Atoh1 and Sox2 and regulates the generation of hair cells.
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1141 (1999). This paper demonstrates that Atoh1 (also known as Math1 ) is essential for the development of hair cells, which is a crucial finding for work focusing on generating hair cells in vitro.
Erkman, L. et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381, 603–606 (1996). References 87 and 88 both demonstrate that POU4F3 (also known as BRN3.1) is essential for the terminal differentiation of hair cells, an important finding for generating hair cells in vitro.
Xiang, M. et al. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc. Natl Acad. Sci. USA 94, 9445–9450 (1997).
Wallis, D. et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 130, 221–232 (2003).
Hu, X. et al. Sonic hedgehog (SHH) promotes the differentiation of mouse cochlear neural progenitors via the Math1–Brn3.1 signaling pathway in vitro. J. Neurosci. Res. 88, 927–935 (2010).
Masuda, M. et al. Regulation of POU4F3 gene expression in hair cells by 5′ DNA in mice. Neuroscience 197, 48–64 (2011).
Chonko, K. T. et al. Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev. Biol. 381, 401–410 (2013).
Zheng, J. L. & Gao, W. Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neurosci. 3, 580–586 (2000). This paper is the first to demonstrate that overexpression of Atoh1 can induce the production of extra hair cells in the inner ear.
Kawamoto, K., Ishimoto, S., Minoda, R., Brough, D. E. & Raphael, Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 23, 4395–4400 (2003).
Gubbels, S. P., Woessner, D. W., Mitchell, J. C., Ricci, A. J. & Brigande, J. V. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537–541 (2008).
Izumikawa, M. et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Med. 11, 271–276 (2005). This study shows that the overexpression of Atoh1 in the cochlea of deaf guinea pigs leads to the generation of hair cells and to improved hearing function.
Yang, S. M. et al. Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLoS ONE 7, e46355 (2012).
Baker, K., Brough, D. E. & Staecker, H. Repair of the vestibular system via adenovector delivery of Atoh1: a potential treatment for balance disorders. Adv. Otorhinolaryngol 66, 52–63 (2009).
Schlecker, C. et al. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 18, 884–890 (2011).
Liu, Z. et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells by Atoh1 ectopic expression. J. Neurosci. 32, 6600–6610 (2012).
Cai, T., Seymour, M. L., Zhang, H., Pereira, F. A. & Groves, A. K. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neurosci. 33, 10110–10122 (2013).
Izumikawa, M., Batts, S. A., Miyazawa, T., Swiderski, D. L. & Raphael, Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear. Res. 240, 52–56 (2008).
Vandenberghe, L. H. & Auricchio, A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 19, 162–168 (2012).
Akil, O. et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75, 283–293 (2012). In this study, it is demonstrated that hearing function can be restored in VGLUT3 knockout mice by reintroducing VGLUT3 cDNA into inner hair cells using AAV viral vectors.
Luebke, A. E., Rova, C., Von Doersten, P. G. & Poulsen, D. J. Adenoviral and AAV-mediated gene transfer to the inner ear: role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv. Otorhinolaryngol. 66, 87–98 (2009).
Driver, E. C., Sillers, L., Coate, T. M., Rose, M. F. & Kelley, M. W. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol. 376, 86–98 (2013).
Mizutari, K. et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58–69 (2013).
Shi, F., Cheng, Y. F., Wang, X. L. & Edge, A. S. β-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J. Biol. Chem. 285, 392–400 (2010).
Jacques, B. E. et al. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 139, 4395–4404 (2012).
Shi, F. et al. β-catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 34, 6470–6479 (2014).
Munnamalai, V. & Fekete, D. M. Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 24, 480–489 (2013).
Roberson, D. W., Alosi, J. A. & Cotanche, D. A. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J. Neurosci. Res. 78, 461–471 (2004).
Lowenheim, H. et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl Acad. Sci. USA 96, 4084–4088 (1999).
Chen, P. & Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581–1590 (1999).
Sage, C. et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 307, 1114–1118 (2005).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Bramhall, N. F., Shi, F., Arnold, K., Hochedliner, K. & Edge, A. S. B. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports 2, 311–322 (2014). This study demonstrates that LGR5-positive supporting cells in the neonatal cochlea can differentiate into hair cells following ototoxic damage.
Chai, R. et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455–469 (2011).
Burns, J. C. & Corwin, J. T. A historical to present-day account of efforts to answer the question: 'what puts the brakes on mammalian hair cell regeneration?'. Hear. Res. 297, 52–67 (2013).
Walters, B. J. & Zuo, J. Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration. Hear. Res. 297, 68–83 (2013).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Oshima, K. et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 (2010). This paper reports that hair cell-like cells can be generated in vitro by the transdifferentiation of embryonic stem cell and induced pluripotent stem cells.
Koehler, K. R., Mikosz, A. M., Molosh, A. I., Patel, D. & Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013). This manuscript describes a new method for the generation of inner ear sensory epithelia containing hair cells in vitro using three- dimensional cultures. The yield of hair cells seems much larger than in previous attempts to generate hair cells.
Malgrange, B. et al. Proliferative generation of mammalian auditory hair cells in culture. Mech. Dev. 112, 79–88 (2002).
Li, H., Liu, H. & Heller, S. Pluripotent stem cells from the adult mouse inner ear. Nature Med. 9, 1293–1299 (2003).
Chen, W. et al. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells 27, 1196–1204 (2009).
Hu, Z. et al. Generation of human inner ear prosensory-like cells via epithelial-to-mesenchymal transition. Regen Med. 7, 663–673 (2012).
Li, H., Roblin, G., Liu, H. & Heller, S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl Acad. Sci. USA 100, 13495–13500 (2003).
Okano, T. & Kelley, M. W. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif. 16, 4–18 (2012).
Chen, W. et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490, 278–282 (2012). This study describes the in vitro generation of otic neuroprogenitors that can differentiate into hair cells and sensory neurons. Upon implantation of the progenitors in a model of auditory neuropathy, auditory function was substantially improved.
Hirose, K. & Liberman, M. C. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 4, 339–352 (2003).
Petrs-Silva, H. & Linden, R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin. Ophthalmol. 8, 127–136 (2014).
Sacheli, R., Delacroix, L., Vandenackerveken, P., Nguyen, L. & Malgrange, B. Gene transfer in inner ear cells: a challenging race. Gene Ther. 20, 237–247 (2013).
Sun, H., Huang, A. & Cao, S. Current status and prospects of gene therapy for the inner ear. Hum. Gene Ther. 22, 1311–1322 (2011).
Maeda, Y., Fukushima, K., Nishizaki, K. & Smith, R. J. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum. Mol. Genet. 14, 1641–1650 (2005).
Lentz, J. J. et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nature Med. 19, 345–350 (2013). This study describes how antisense technology can be used to suppress a splice-site mutation to recover auditory function in an animal model for human deafness.
Miwa, T., Minoda, R., Ise, M., Yamada, T. & Yumoto, E. Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Mol. Ther. 21, 1142–1150 (2013).
Rivera, T., Sanz, L., Camarero, G. & Varela-Nieto, I. Drug delivery to the inner ear: strategies and their therapeutic implications for sensorineural hearing loss. Curr. Drug Deliv. 9, 231–242 (2012).
Schuknecht, H. F. Pathology of the Ear. (Harvard Univ. Press, 1974).
Nakamoto, Y., Iino, Y. & Kodera, K. [Temporal bone histopathology of noise-induced hearing loss]. Nihon Jibiinkoka Gakkai Kaiho 108, 172–181 (2005).
Wang, Y., Hirose, K. & Liberman, M. C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 3, 248–268 (2002).
Yan, C. & Higgins, P. J. Drugging the undruggable: transcription therapy for cancer. Biochim. Biophys. Acta 1835, 76–85 (2013).
Wulff, H., Castle, N. A. & Pardo, L. A. Voltage-gated potassium channels as therapeutic targets. Nature Rev. Drug Discov. 8, 982–1001 (2009).
Amabile, C. M. & Vasudevan, A. Ezogabine: a novel antiepileptic for adjunctive treatment of partial-onset seizures. Pharmacotherapy 33, 187–194 (2013).
Shiroshita-Takeshita, A., Sakabe, M., Haugan, K., Hennan, J. K. & Nattel, S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation 115, 310–318 (2007).
Cao, Y. & Zheng, O. J. Tonabersat for migraine prophylaxis: a systematic review. Pain Physician 17, 1–8 (2014).
O'Carroll, S. J. et al. The use of connexin-based therapeutic approaches to target inflammatory diseases. Methods Mol. Biol. 1037, 519–546 (2013).
Waris, G. & Ahsan, H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog. 5, 14 (2006).
Daniel, E. Noise and hearing loss: a review. J. Sch. Health 77, 225–231 (2007).
Wong, A. C. et al. Post exposure administration of A(1) adenosine receptor agonists attenuates noise-induced hearing loss. Hear. Res. 260, 81–88 (2010).
Fetoni, A. R. et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience 169, 1575–1588 (2010).
Kopke, R. D. et al. NAC for noise: from the bench top to the clinic. Hear. Res. 226, 114–125 (2007).
Bielefeld, E. C. et al. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 127, 914–919 (2007).
Ohlemiller, K. K. et al. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol 4, 237–246 (1999).
McFadden, S. L., Ding, D., Reaume, A. G., Flood, D. G. & Salvi, R. J. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol. Aging 20, 1–8 (1999).
Keithley, E. M. et al. Cu/Zn superoxide dismutase and age-related hearing loss. Hear. Res. 209, 76–85 (2005).
Kashio, A. et al. Effect of vitamin C depletion on age-related hearing loss in SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 390, 394–398 (2009).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Seidman, M. D. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 110, 727–738 (2000).
Seidman, M. D., Khan, M. J., Tang, W. X. & Quirk, W. S. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol. Head Neck Surg. 127, 138–144 (2002).
Someya, S. et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl Acad. Sci. USA 106, 19432–19437 (2009).
Heman-Ackah, S. E., Juhn, S. K., Huang, T. C. & Wiedmann, T. S. A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol. Head Neck Surg. 143, 429–434 (2010).
Markaryan, A., Nelson, E. G. & Hinojosa, R. Detection of mitochondrial DNA deletions in the cochlea and its structural elements from archival human temporal bone tissue. Mutat. Res. 640, 38–45 (2008).
Meltser, I., Tahera, Y. & Canlon, B. Differential activation of mitogen-activated protein kinases and brain-derived neurotrophic factor after temporary or permanent damage to a sensory system. Neuroscience 165, 1439–1446 (2010).
Wang, J. et al. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J. Neurosci. 23, 8596–8607 (2003).
Shim, H. J., Kang, H. H., Ahn, J. H. & Chung, J. W. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta Otolaryngol. 129, 233–238 (2009).
Ahn, J. H., Kang, H. H., Kim, Y. J. & Chung, J. W. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem. Biophys. Res. Commun. 335, 485–490 (2005).
Suckfuell, M., Canis, M., Strieth, S., Scherer, H. & Haisch, A. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 127, 938–942 (2007).
Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 5, e996 (2014).
Fischer, U. & Schulze-Osthoff, K. New approaches and therapeutics targeting apoptosis in disease. Pharmacol. Rev. 57, 187–215 (2005).
Rybak, L. P. & Whitworth, C. A. Ototoxicity: therapeutic opportunities. Drug Discov. Today 10, 1313–1321 (2005).
Sha, S. H., Qiu, J. H. & Schacht, J. Aspirin to prevent gentamicin-induced hearing loss. N. Engl. J. Med. 354, 1856–1857 (2006).
Feldman, L. et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 72, 359–363 (2007).
Kharkheli, E., Kevanishvili, Z., Maglakelidze, T., Davitashvili, O. & Schacht, J. Does vitamin E prevent gentamicin-induced ototoxicity? Georgian Med. News 146, 14–17 (2007).
Hainrichson, M. et al. Branched aminoglycosides: biochemical studies and antibacterial activity of neomycin B derivatives. Bioorg. Med. Chem. 13, 5797–5807 (2005).
Armstrong, E. S. & Miller, G. H. Combating evolution with intelligent design: the neoglycoside ACHN-490. Curr. Opin. Microbiol. 13, 565–573 (2010).
Pokrovskaya, V., Nudelman, I., Kandasamy, J. & Baasov, T. Aminoglycosides redesign strategies for improved antibiotics and compounds for treatment of human genetic diseases. Methods Enzymol. 478, 437–462 (2010).
Shin, Y. S. et al. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: in vitro and in vivo study. Neuroscience 232, 1–12 (2012).
Ozkiris, M., Kapusuz, Z., Karacavus, S. & Saydam, L. The effects of lycopene on cisplatin-induced ototoxicity. Eur. Arch. Otorhinolaryngol 270, 3027–3033 (2013).
Sagit, M., Korkmaz, F., Akcadag, A. & Somdas, M. A. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur. Arch. Otorhinolaryngol. 270, 2231–2237 (2013).
Simsek, G. et al. Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur. Arch. Otorhinolaryngol. 270, 1789–1793 (2013).
Shin, Y. S. et al. Novel synthetic protective compound, KR-22335, against cisplatin-induced auditory cell death. J. Appl. Toxicol. 34, 191–204 (2014).
Brock, P. R. et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology Boston ototoxicity scale. J. Clin. Oncol. 30, 2408–2417 (2012).
Doolittle, N. D. et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin. Cancer Res. 7, 493–500 (2001).
Neuwelt, E. A. et al. Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood–brain-barrier disruption. Pediatr. Blood Cancer 47, 174–182 (2006).
Reid, F. M., Vernham, G. A. & Jacobs, H. T. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum. Mutat. 3, 243–247 (1994).
Bharadwaj, H. M., Verhulst, S., Shaheen, L., Liberman, M. C. & Shinn-Cunningham, B. G. Cochlear neuropathy and the coding of supra-threshold sound. Front. Syst. Neurosci. 8, 26 (2014).
Fritzsch, B., Pirvola, U. & Ylikoski, J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 295, 369–382 (1999).
Rabbitt, R. D. & Brownell, W. E. Efferent modulation of hair cell function. Otolaryngol. Head Neck Surg. 19, 376–381 (2011).
Maison, S. F., Luebke, A. E., Liberman, M. C. & Zuo, J. Efferent protection from acoustic injury is mediated via α9 nicotinic acetylcholine receptors on outer hair cells. J. Neurosci. 22, 10838–10846 (2002). This study demonstrates the importance of efferent neurons, which connect to OHCs, for the prevention of NIHL.
Taranda, J. et al. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol. 7, e18 (2009).
Liberman, M. C., Liberman, L. D. & Maison, S. F. Efferent feedback slows cochlear aging. J. Neurosci. 34, 4599–4607 (2014).
Owens, K. N. et al. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 4, e1000020 (2008). This study describes the usefulness of the lateral line organ of zebrafish, which contains hair cells that sense fluid motion, for identifying genetic mutations and pharmacological compounds that prevent hair cell death induced by ototoxic agents.
Ou, H. C. et al. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J. Assoc. Res. Otolaryngol. 10, 191–203 (2009).
Ton, C. & Parng, C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear. Res. 208, 79–88 (2005).
Chiu, L. L., Cunningham, L. L., Raible, D. W., Rubel, E. W. & Ou, H. C. Using the zebrafish lateral line to screen for ototoxicity. J. Assoc. Res. Otolaryngol. 9, 178–190 (2008).
Hirose, Y., Simon, J. A. & Ou, H. C. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J. Assoc. Res. Otolaryngol. 12, 719–728 (2011).
Namdaran, P., Reinhart, K. E., Owens, K. N., Raible, D. W. & Rubel, E. W. Identification of modulators of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 32, 3516–3528 (2012).
Xiong, W., Wagner, T., Yan, L., Grillet, N. & Müller, U. Using injectoporation to deliver genes to mechanosensory hair cells. Nature Protoc. 9, 2438–2449 (2014). This study describes a new method that enables the culturing of explants of the inner-ear sensory epithelia that are suitable for gene transfer into hair cells and for analysing the functional properties of hair cells by electrophysiology.
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Ronaghi, M. et al. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 23, 1275–1284 (2014).
Baguley, D., McFerran, D. & Hall, D. Tinnitus. Lancet 382, 1600–1607 (2013).
Langguth, B., Kreuzer, P. M., Kleinjung, T. & De Ridder, D. Tinnitus: causes and clinical management. Lancet Neurol. 12, 920–930 (2013).
Berlinger, N. T. Meniere's disease: new concepts, new treatments. Minn. Med. 94, 33–36 (2011).
Semaan, M. T., Alagramam, K. N. & Megerian, C. A. The basic science of Meniere's disease and endolymphatic hydrops. Otolaryngol. Head Neck Surg. 13, 301–307 (2005).
Li, J. D. et al. Panel 4: recent advances in otitis media in molecular biology, biochemistry, genetics, and animal models. Otolaryngol. Head Neck Surg. 148, E52–E63 (2013).
Grillet, N. et al. Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans. Am. J. Hum. Genet. 85, 328–337 (2009).
Schwander, M. et al. A novel allele of myosin VIIa reveals a critical function for the C-terminal FERM domain for melanosome transport in retinal pigment epithelial cells. J. Neurosci. 29, 15810–15818 (2009).
Koehler, K. R. & Hashino, E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nature Protoc. 9, 1229–1244 (2014).
Walsh, T. et al. From flies' eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc. Natl Acad. Sci. USA 99, 7518–7523 (2002).
Melchionda, S. et al. MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am. J. Hum. Genet. 69, 635–640 (2001).
Ahmed, Z. M. et al. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am. J. Hum. Genet. 72, 1315–1322 (2003).
Weil, D. et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374, 60–61 (1995).
Liu, X. Z. et al. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nature Genet. 16, 188–190 (1997).
Weil, D. et al. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nature Genet. 16, 191–193 (1997).
Donaudy, F. et al. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am. J. Hum. Genet. 72, 1571–1577 (2003).
Wang, A. et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280, 1447–1451 (1998).
Zhu, M. et al. Mutations in the γ-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am. J. Hum. Genet. 73, 1082–1091 (2003).
van Wijk, E. et al. A mutation in the gamma actin 1 (ACTG1) gene causes autosomal dominant hearing loss (DFNA20/26). J. Med. Genet. 40, 879–884 (2003).
Khan, S. Y. et al. Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus. Hum. Mutat. 28, 417–423 (2007).
Shahin, H. et al. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am. J. Hum. Genet. 78, 144–152 (2006).
Riazuddin, S. et al. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am. J. Hum. Genet. 78, 137–143 (2006).
Li, Y. et al. Mutations in TPRN cause a progressive form of autosomal-recessive nonsyndromic hearing loss. Am. J. Hum. Genet. 86, 479–484 (2010).
Rehman, A. U. et al. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am. J. Hum. Genet. 86, 378–388 (2010).
Naz, S. et al. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J. Med. Genet. 41, 591–595 (2004).
Verpy, E. et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nature Genet. 26, 51–55 (2000).
Bitner-Glindzicz, M. et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nature Genet. 26, 56–60 (2000).
Ouyang, X. M. et al. Mutations in the alternatively spliced exons of USH1C cause non-syndromic recessive deafness. Hum. Genet. 111, 26–30 (2002).
Ahmed, Z. M. et al. Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USHIC. Hum. Genet. 110, 527–531 (2002).
Mburu, P. et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nature Genet. 34, 421–428 (2003).
Ebermann, I. et al. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum. Genet. 121, 203–211 (2007).
Mustapha, M. et al. A novel locus for Usher syndrome type I, USH1G, maps to chromosome 17q24-25. Hum. Genet. 110, 348–350 (2002).
Weil, D. et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum. Mol. Genet. 12, 463–471 (2003).
Schraders, M. et al. Mutations in PTPRQ are a cause of autosomal-recessive nonsyndromic hearing impairment DFNB84 and associated with vestibular dysfunction. Am. J. Hum. Genet. 86, 604–610 (2010).
Weston, M. D., Luijendijk, M. W., Humphrey, K. D., Moller, C. & Kimberling, W. J. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 74, 357–366 (2004).
Verpy, E. et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nature Genet. (2001).
Borck, G. et al. Loss-of-function mutations of ILDR1 cause autosomal-recessive hearing impairment DFNB42. Am. J. Hum. Genet. 88, 127–137 (2011).
Bork, J. M. et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet. 68, 26–37 (2001).
Bolz, H. et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nature Genet. 27, 108–112 (2001).
Ahmed, Z. M. et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 69, 25–34 (2001).
Alagramam, K. N. et al. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet. 10, 1709–1718 (2001).
Kurima, K. et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nature Genet. 30, 277–284 (2002).
Kalay, E. et al. Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum. Mutat. 27, 633–639 (2006).
Shabbir, M. I. et al. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J. Med. Genet. 43, 634–640 (2006).
Kelsell, D. P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 (1997).
Liu, X. Z. et al. Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum. Mol. Genet. 9, 63–67 (2000).
Xia, J. H. et al. Mutations in the gene encoding gap junction protein β-3 associated with autosomal dominant hearing impairment. Nature Genet. 20, 370–373 (1998).
del Castillo, I. et al. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N. Engl. J. Med. 346, 243–249 (2002).
Grifa, A. et al. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nature Genet. 23, 16–18 (1999).
Wilcox, E. R. et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104, 165–172 (2001).
Riazuddin, S. et al. Tricellulin is a tight-junction protein necessary for hearing. Am. J. Hum. Genet. 79, 1040–1051 (2006).
Walsh, T. et al. Genomic duplication and overexpression of TJP2/ZO-2 leads to altered expression of apoptosis genes in progressive nonsyndromic hearing loss DFNA51. Am. J. Hum. Genet. 87, 101–109 (2010).
Neyroud, N. et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nature Genet. 15, 186–189 (1997).
Kubisch, C. et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96, 437–446 (1999).
Tyson, J. et al. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum. Mol. Genet. 6, 2179–2185 (1997).
Schulze-Bahr, E. et al. KCNE1 mutations cause jervell and Lange-Nielsen syndrome. Nature Genet. 17, 267–268 (1997).
Reichold, M. et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc. Natl Acad. Sci. USA 107, 14490–14495 (2010).
Collin, R. W. et al. Mutations of ESRRB encoding estrogen-related receptor beta cause autosomal-recessive nonsyndromic hearing impairment DFNB35. Am. J. Hum. Genet. 82, 125–138 (2008).
Wayne, S. et al. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum. Mol. Genet. 10, 195–200 (2001).
Vahava, O. et al. Mutation in transcription factor POU4F3 associated with inherited progressive hearing loss in humans. Science 279, 1950–1954 (1998).
Mencia, A. et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nature Genet. 41, 609–613 (2009).
von Ameln, S. et al. A mutation in PNPT1, encoding mitochondrial-RNA-import protein PNPase, causes hereditary hearing loss. Am. J. Hum. Genet. 91, 919–927 (2012).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
van den Ouweland, J. M. et al. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nature Genet. 1, 368–371 (1992).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61, 931–937 (1990).
Zeviani, M. et al. A MERRF/MELAS overlap syndrome associated with a new point mutation in the mitochondrial DNA tRNA(Lys) gene. Eur. J. Hum. Genet. 1, 80–87 (1993).
Kameoka, K. et al. Novel mitochondrial DNA mutation in tRNA(Lys) (8296A—>G) associated with diabetes. Biochem. Biophys. Res. Commun. 245, 523–527 (1998).
Jaksch, M. et al. Progressive myoclonus epilepsy and mitochondrial myopathy associated with mutations in the tRNA(Ser(UCN)) gene. Ann. Neurol. 44, 635–640 (1998).
Tiranti, V. et al. Maternally inherited hearing loss, ataxia and myoclonus associated with a novel point mutation in mitochondrial tRNASer(UCN) gene. Hum. Mol. Genet. 4, 1421–1427 (1995).
Hao, H., Bonilla, E., Manfredi, G., DiMauro, S. & Moraes, C. T. Segregation patterns of a novel mutation in the mitochondrial tRNA glutamic acid gene associated with myopathy and diabetes mellitus. Am. J. Hum. Genet. 56, 1017–1025 (1995).
Prezant, T. R. et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nature Genet. 4, 289–294 (1993).
Ahmed, Z. M. et al. Functional null mutations of MSRB3 encoding methionine sulfoxide reductase are associated with human deafness DFNB74. Am. J. Hum. Genet. 88, 19–29 (2011).
Yasunaga, S. et al. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nature Genet. 21, 363–369 (1999).
Du, X. et al. A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc. Natl Acad. Sci. USA 105, 14609–14614 (2008).
Ahmed, Z. M. et al. Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans. Nature Genet. 40, 1335–1340 (2008).
Verhoeven, K. et al. Mutations in the human α-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nature Genet. 19, 60–62 (1998).
Zwaenepoel, I. et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl Acad. Sci. USA 99, 6240–6245 (2002).
Robertson, N. G. et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nature Genet. 20, 299–303 (1998).
Yariz, K. O. et al. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am. J. Hum. Genet. 91, 872–882 (2012).
Schraders, M. et al. Mutations of the gene encoding otogelin are a cause of autosomal-recessive nonsyndromic moderate hearing impairment. Am. J. Hum. Genet. 91, 883–889 (2012).
Barker, D. F. et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248, 1224–1227 (1990).
Mochizuki, T. et al. Identification of mutations in the α3(IV) and α4(IV) collagen genes in autosomal recessive Alport syndrome. Nature Genet. 8, 77–81 (1994).
McGuirt, W. T. et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nature Genet. 23, 413–419 (1999).
Van Laer, L. et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum. Mutat. 27, 786–795 (2006).
Pawelczyk, M. et al. Analysis of gene polymorphisms associated with K+ ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann. Hum. Genet. 73, 411–421 (2009).
Konings, A. et al. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum. Mol. Genet. 16, 1872–1883 (2007).
Konings, A. et al. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann. Hum. Genet. 73, 215–224 (2009).
Konings, A. et al. Variations in HSP70 genes associated with noise-induced hearing loss in two independent populations. Eur. J. Hum. Genet. 17, 329–335 (2009).
Yang, M. et al. Association of hsp70 polymorphisms with risk of noise-induced hearing loss in Chinese automobile workers. Cell Stress Chaperones 11, 233–239 (2006).
Peters, U. et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs 11, 639–643 (2000).
Oldenburg, J., Kraggerud, S. M., Cvancarova, M., Lothe, R. A. & Fossa, S. D. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J. Clin. Oncol. 25, 708–714 (2007).
Xu, X. et al. Genetic polymorphism of copper transporter protein 1 is related to platinum resistance in Chinese non-small cell lung carcinoma patients. Clin. Exp. Pharmacol. Physiol. 39, 786–792 (2012).
Caronia, D. et al. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenom. J. 9, 347–353 (2009).
Noben-Trauth, K., Zheng, Q. Y. & Johnson, K. R. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nature Genet. 35, 21–23 (2003).
Johnson, K. R., Gagnon, L. H., Longo-Guess, C. & Kane, K. L. Association of a citrate synthase missense mutation with age-related hearing loss in A/J mice. Neurobiol. Aging 33, 1720–1729 (2012).
Charizopoulou, N. et al. Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nature Commun. 2, 201 (2011).
Shin, J. B. et al. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J. Neurosci. 30, 9683–9694 (2010).
Skradski, S. L. et al. A novel gene causing a mendelian audiogenic mouse epilepsy. Neuron 31, 537–544 (2001).
Johnson, K. R., Zheng, Q. Y., Bykhovskaya, Y., Spirina, O. & Fischel-Ghodsian, N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nature Genet. 27, 191–194 (2001).
Acknowledgements
This work was supported by NIH grants R01 DC002368 (P.B-G.), R01 DC011034 (P.B-G.), R01 DC005965 (U.M.) and RO1 DC007704 (U.M.); fellowship support from the Hearing Health Foundation (P.B-G); the Dorris Neuroscience Center (U.M.), the Skaggs Institute for Chemical Biololgy (U.M.) and the California Institute of Regenerative Medicine (U.M.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Nonsyndromic deafness
-
A genetic form of hearing loss that affects hearing function without any other symptoms in different tissues and organs.
- Syndromic deafness
-
A genetic form of hearing loss that affects not only hearing but also the function of other tissues and organs.
- Ototoxic
-
A substance that is toxic to the ear, such as certain antibiotics and chemotherapy agents.
- Tinnitus
-
Perception of a ringing tone within the ear without an external sound trigger.
- Cochlear implants
-
Electrical medical devices that are implanted into the cochlea as a substitute for the function of damaged hair cells.
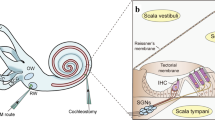
- Organ of Corti
-
The end-organ in the inner ear that functions in sound perception.
- Outer hair cells
-
(OHCs). Sensory cells for sound perception that are required to amplify input sound signals for their subsequent detection and processing by inner hair cells.
- Inner hair cells
-
Sensory cells that transmit sound information via neurons to the nervous system.
- Tectorial membrane
-
An acellular membrane of the inner ear that covers the mechanically sensitive hair bundles of hair cells and is required for the transmission of mechanical signals that are evoked by sound stimuli.
- Basilar membrane
-
A stiff acellular structure of the inner ear that underlies the sensory epithelium and resonates in response to sound-induced mechanical signals.
- Tympanic membrane
-
A membrane that separates the external from the middle ear, also referred to as the eardrum.
- Ossicles
-
The three bones of the middle ear that transmit sound information from the external ear to the cochlea in the inner ear.
- Synapses
-
Structural specializations that allow signal transmission between neurons or from sensory cells, such as hair cells, to neurons.
- Hypomorphic alleles
-
Alleles of genes that reduce the activity of the gene or of its gene product.
- Reactive oxygen species
-
(ROS). Chemically reactive molecules that contain oxygen and have important functions for cell signalling, but in excessive amounts can cause tissue damage.
- Endolymph
-
The fluid within the membranous labyrinth of the inner ear that bathes the stereocilia of hair cells.
- Gap junctions
-
Specialized intracellular junctions that connect the cytoplasm of two cells and enable the passage of molecules and ions between them.
- Fibrocytes
-
Mesenchymal cells that are distributed throughout the inner ear and are thought to be involved in K+ recycling.
- Stria vascularis
-
A secretory epithelium of the inner ear that produces and secretes endolymph and also sets the endocochlear potential.
- Temporary threshold shift
-
A temporary, recoverable loss in sensitivity to sound.
- Permanent threshold shift
-
Permanent loss in the sensitivity to sound.
- Aminoglycoside antibiotics
-
Antibacterial drugs directed against Gram-negative bacteria that inhibit protein synthesis.
- Apoptosis
-
Programmed cell death that relies on the activation of a cascade of events, which are genetically encoded.
- Single nucleotide polymorphisms
-
(SNPs). Variation in DNA sequences in which a single nucleotide differs in the genome between members of a species.
- Audiogram
-
A graph that represents the response of the auditory system to a range of standardized frequencies and intensities, which is used to determine the sensitivity of the auditory system to sound.
- Genome-wide association studies
-
(GWAS). A methodology that scans markers across the entire genome to identify genetic variations that are associated with genetic traits, including susceptibility to hearing loss.
- Serotypes
-
Groups of viruses or microorganisms that can be distinguished by shared specific antigens, which are determined by serological tests.
- Pillar cells
-
Supporting cells within the sensory epithelium of the inner ear that form the walls of a fluid-filled tunnel between the inner and outer hair cells.
- Deiters' cells
-
Supporting cells within the sensory epithelium of the inner ear that sit on the basilar membrane and hold the base of outer hair cells. They also form apical processes that extend next to the apical surfaces of outer hair cells.
- Auditory brain stem response
-
Electrical potentials that are measured with electrodes placed on the scalp while the ear is stimulated with sound of defined intensity and frequency using a loudspeaker.
Rights and permissions
About this article
Cite this article
Müller, U., Barr-Gillespie, P. New treatment options for hearing loss. Nat Rev Drug Discov 14, 346–365 (2015). https://doi.org/10.1038/nrd4533
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4533
This article is cited by
-
Treatment of monogenic and digenic dominant genetic hearing loss by CRISPR-Cas9 ribonucleoprotein delivery in vivo
Nature Communications (2023)
-
Revealing the contribution of basilar membrane’s biological activity to the mechanism of the cochlear phonosensitive amplification
Applied Mathematics and Mechanics (2023)
-
Apigenin alleviates neomycin-induced oxidative damage via the Nrf2 signaling pathway in cochlear hair cells
Frontiers of Medicine (2022)
-
Towards maturation of human otic hair cell–like cells in pluripotent stem cell–derived organoid transplants
Cell and Tissue Research (2021)
-
Distortion Product Otoacoustic Emission (DPOAE) Growth in Aging Ears with Clinically Normal Behavioral Thresholds
Journal of the Association for Research in Otolaryngology (2021)