Key Points
-
Hypoxia-inducible factors (HIFs) were originally described as transcription factors that promote transcriptional responses during hypoxia adaptation (for example, increased erythropoietin release following blood loss or during hypoxia). More recently, it has been appreciated that HIFs are also active during a wider range of disease conditions, including inflammation and ischaemia.
-
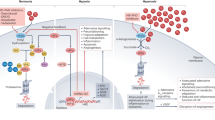
During inflammatory or ischaemic conditions, HIFs are stabilized and are transcriptionally active. In many instances, they promote a gene programme that dampens acute inflammation and that promotes resolution of injury.
-
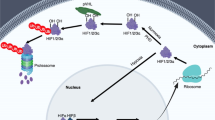
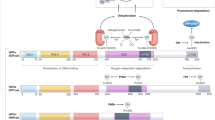
During normoxic conditions, HIFs are inactive, as they are targeted for proteasomal degradation by prolyl hydroxylases (PHDs). During hypoxic conditions, PHDs are inactive, and HIFs become stabilized and transcriptionally active. Pharmacologically, HIFs can be stabilized by small molecules that function to inhibit PHDs. Such compounds have recently gained great interest.
-
Using pharmacological HIF activators (mostly PHD inhibitors), a HIF-dependent gene expression programme can be activated with the goal to dampen inflammation while promoting the resolution of injury. Examples for diseases for which there is experimental evidence indicating a protective role for HIF activators include inflammatory bowel disease (IBD), acute lung injury (ALI), myocardial ischaemia and reperfusion injury, acute kidney injury and organ transplantation.
-
Other studies have identified pharmacological approaches to inhibit HIFs. Such approaches could hold therapeutic potential in the treatment of cancer or fibrosis (for example, renal fibrosis).
-
Several companies have developed orally available PHD inhibitors that promote HIF stabilization under normoxic conditions. Such compounds are currently examined in clinical trials, for example, for the treatment of renal anaemia or perioperative ischaemia and reperfusion injury.
-
Although most clinical and experimental studies so far have found that short-term PHD inhibitor treatment is safe, careful monitoring for potentially detrimental side effects of PHD inhibitors will be crucial, particularly for their chronic application in patients. Potential side effects could include liver injury, sepsis, increased erythropoiesis or cancer.
-
Challenges for the field include a better understanding of the specific roles for individual HIF isoforms (for example, HIF1α and HIF2α), the design of pharmacological approaches to specifically target individual PHDs or individual HIFs, and pharmacological delivery systems that allow tissue-specific delivery of HIF activators (for example, via inhalation to alveolar epithelial cells or approaches to specifically target intestinal epithelial cells).
-
We anticipate that, in the near future, HIF activators will be used routinely in a clinical setting for organ protection in patients experiencing ischaemia and reperfusion injury, as well as to promote the resolution of inflammation during acute or chronic inflammatory disease states such as IBD or ALI.
Abstract
Hypoxia-inducible factors (HIFs) are stabilized during adverse inflammatory processes associated with disorders such as inflammatory bowel disease, pathogen infection and acute lung injury, as well as during ischaemia–reperfusion injury. HIF stabilization and hypoxia-induced changes in gene expression have a profound impact on the inflamed tissue microenvironment and on disease outcomes. Although the mechanism that initiates HIF stabilization may vary, the final molecular steps that control HIF stabilization converge on a set of oxygen-sensing prolyl hydroxylases (PHDs) that mark HIFs for proteasomal degradation. PHDs are therefore promising therapeutic targets. In this Review, we discuss the emerging potential and associated challenges of targeting the PHD–HIF pathway for the treatment of inflammatory and ischaemic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, C. T. & McElwain, J. C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25, 272–279 (2010).
Semenza, G. L. & Wang, G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 (1992).
Wang, G., Jiang, B., Rue, E. & Semenza, G. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Firth, J. D., Ebert, B. L., Pugh, C. W. & Ratcliffe, P. J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc. Natl Acad. Sci. USA 91, 6496–6500 (1994).
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994). Landmark paper that identified binding of a hypoxia-inducible transcription factor, which binds to the erythropietin promoter and was subsequently named HIF.
Taylor, C. T. Interdependent roles for hypoxia inducible factor and nuclear factor-κB in hypoxic inflammation. J. Physiol. 586, 4055–4059 (2008).
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nature Rev. Mol. Cell Biol. 5, 343–354 (2004).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Semenza, G. L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 365, 537–547 (2011).
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion — from mechanism to translation. Nature Med. 17, 1391–1401 (2011).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Zhang, H. et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc. Natl Acad. Sci. USA 105, 19579–19586 (2008).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Semenza, G. L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24, 97–106 (2009).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Tanimoto, K., Makino, Y., Pereira, T. & Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19, 4298–4309 (2000).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). Landmark paper that identified mammalian PHDs in their functional role of regulating the stability of HIF.
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Murray, L. W. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002).
Ema, M. et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl Acad. Sci. USA 94, 4273–4278 (1997).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 7, 205–213 (1998).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J. Biol. Chem. 277, 32405–32408 (2002).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Bruick, R. K. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 17, 2614–2623 (2003).
Mikus, P. & Zundel, W. COPing with hypoxia. Semin. Cell Dev. Biol. 16, 462–473 (2005).
Boh, B. K., Smith, P. G. & Hagen, T. Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J. Mol. Biol. 409, 136–145 (2011).
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009).
Khoury, J., Ibla, J. C., Neish, A. S. & Colgan, S. P. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J. Clin. Invest. 117, 703–711 (2007).
Ehrentraut, S. F. et al. Central role for endothelial human deneddylase-1/SENP8 in fine-tuning the vascular inflammatory response. J. Immunol. 190, 392–400 (2013).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Colgan, S. P. & Eltzschig, H. K. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 74, 153–175 (2012).
Colgan, S. P. & Taylor, C. T. Hypoxia: an alarm signal during intestinal inflammation. Nature Rev. Gastroenterol. Hepatol 7, 281–287 (2010).
Karhausen, J. O. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004).
Clambey, E. T. et al. Hypoxia-inducible factor-1α-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl Acad. Sci. USA 109, E2784–E2793 (2012).
Peyssonnaux, C. et al. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J. Immunol. 178, 7516–7519 (2007).
Kuhlicke, J., Frick, J. S., Morote-Garcia, J. C., Rosenberger, P. & Eltzschig, H. K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2, e1364 (2007).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Werth, N. et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 5, e11576 (2010).
Hartmann, H. et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134, 756–767 (2008).
Kempf, V. A. et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111, 1054–1062 (2005).
Haeberle, H. A. et al. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE 3, e3352 (2008).
Nizet, V. & Johnson, R. S. Interdependence of hypoxic and innate immune responses. Nature Rev. Immunol. 9, 609–617 (2009).
Peyssonnaux, C. et al. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 115, 1806–1815 (2005).
Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 453, 807–811 (2008).
Grocott, M. P. et al. Arterial blood gases and oxygen content in climbers on Mount Everest. N. Engl. J. Med. 360, 140–149 (2009).
Hartmann, G. et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12, 246–252 (2000).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Schmit, M. A. et al. Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation 35, 566–573 (2012).
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl Acad. Sci. USA 103, 18154–18159 (2006). An important study that identified a functional role of PHDs in the stabilization of nuclear factor κB under hypoxic conditions.
Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Marks, D. J., Rahman, F. Z., Sewell, G. W. & Segal, A. W. Crohn's disease: an immune deficiency state. Clin. Rev. Allergy Immunol. 38, 20–31 (2010).
Taylor, C. T. & Colgan, S. P. Hypoxia and gastrointestinal disease. J. Mol. Med. 85, 1295–1300 (2007).
Huang, J. S. et al. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn's disease. Clin. Gastroenterol. Hepatol. 2, 690–695 (2004).
Hart, M. L. et al. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol. 186, 4367–4374 (2011).
Xue, X. et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145, 831–841 (2013).
Tambuwala, M. M. et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139, 2093–2101 (2010).
Shah, Y. M. et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134, 2036–2048 (2008).
Aherne, C. M. et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 61, 695–705 (2012).
Frick, J. S. et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol. 182, 4957–4964 (2009).
Eltzschig, H. K., Rivera-Nieves, J. & Colgan, S. P. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets 13, 1267–1277 (2009).
Ehrentraut, H. et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 27, 2207–2219 (2013).
Ehrentraut, H., Westrich, J. A., Eltzschig, H. K. & Clambey, E. T. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS ONE 7, e32416 (2012).
Xiao, C. & Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell 136, 26–36 (2009).
Fraisl, P., Aragones, J. & Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nature Rev. Drug Discov. 8, 139–152 (2009).
Cummins, E. P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Keely, S. et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 22, 114–123 (2013).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Murry, C. E., Jennings, R. B. & Reimer, K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136 (1986).
Kohler, D. et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116, 1784–1794 (2007).
Eckle, T. et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115, 1581–1590 (2007).
Eckle, T. et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am. J. Physiol. Heart Circ. Physiol. 291, H2533–H2540 (2006).
Eltzschig, H. K. Adenosine: an old drug newly discovered. Anesthesiology 111, 904–915 (2009).
Eckle, T., Kohler, D., Lehmann, R., El Kasmi, K. C. & Eltzschig, H. K. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118, 166–175 (2008).
Eltzschig, H. K., Bonney, S. K. & Eckle, T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 19, 345–354 (2013).
Eckle, T. et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Med. 18, 774–782 (2012).
Botker, H. E. et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375, 727–734 (2010).
Cai, Z., Luo, W., Zhan, H. & Semenza, G. L. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc. Natl Acad. Sci. USA 110, 17462–17467 (2013).
Kharbanda, R. K., Nielsen, T. T. & Redington, A. N. Translation of remote ischaemic preconditioning into clinical practice. Lancet 374, 1557–1565 (2009).
Ali, Z. A. et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116 (11 Suppl.), I98–I105 (2007).
Eckle, T. et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 27, 3078–3089 (2013).
Schingnitz, U. et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 184, 5271–5279 (2010).
Eckle, T., Koeppen, M. & Eltzschig, H. K. Role of extracellular adenosine in acute lung injury. Physiology 24, 298–306 (2009).
Eckle, T. et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111, 2024–2035 (2008).
Eckle, T., Grenz, A., Laucher, S. & Eltzschig, H. K. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 118, 3301–3315 (2008).
Dengler, V., Downey, G. P., Tuder, R. M., Eltzschig, H. K. & Schmidt, E. P. Neutrophil intercellular communication in acute lung injury: emerging roles of microparticles and gap junctions. Am. J. Respir. Cell. Mol. Biol. 49, 1–5 (2013).
Ware, L. B. & Matthay, M. A. The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 (2000).
Ranieri, V. M. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–2533 (2012).
Herridge, M. S. et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 364, 1293–1304 (2011).
Sitkovsky, M. & Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nature Rev. Immunol. 5, 712–721 (2005).
Sitkovsky, M. V. et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 (2004).
Thiel, M. et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 3, e174 (2005). An important study indicating that hyperoxic conditions — which may be necessary for the treatment of patients with ALI to maintain oxygenation of different tissues — can be detrimental via inhibition of anti-inflammatory and tissue-protective functions of HIFs.
Eckle, T. et al. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 11, e1001665 (2013).
Kominsky, D. J., Campbell, E. L. & Colgan, S. P. Metabolic shifts in immunity and inflammation. J. Immunol. 184, 4062–4068 (2010).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Kojima, H. et al. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α -deficient chimeric mice. Proc. Natl Acad. Sci. USA 99, 2170–2174 (2002).
Okumura, C. Y. et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J. Mol. Med. 28, 1079–1089 (2012).
Warnecke, C. et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. 17, 1186–1188 (2003).
Schaible, B. et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE. 8, e56491 (2013).
Kelly, C. J. et al. Fundamental role for HIF-1α in constitutive expression of human βdefensin-1. Mucosal Immunol. 6, 6 (2013).
Pazgier, M., Hoover, D. M., Yang, D., Lu, W. & Lubkowski, J. Human β-defensins. Cell. Mol. Life Sci. 63, 1294–1313 (2006).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nature Rev. Immunol. 3, 710–720 (2003).
Schroeder, B. O. et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 (2011).
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276, 5707–5713 (2001).
O'Neil, D. A. et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 (1999).
Zhao, C., Wang, I. & Lehrer, R. I. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396, 319–322 (1996).
Peyrin-Biroulet, L. et al. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc. Natl Acad. Sci. USA 107, 8772–8777 (2010).
Kocsis, A. K. et al. Association of β-defensin 1 single nucleotide polymorphisms with Crohn's disease. Scand. J. Gastroenterol. 43, 299–307 (2008).
Wehkamp, J. et al. Inducible and constitutive β-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 9, 215–223 (2003).
Jurevic, R. J., Bai, M., Chadwick, R. B., White, T. C. & Dale, B. A. Single-nucleotide polymorphisms (SNPs) in human β-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J. Clin. Microbiol. 41, 90–96 (2003).
Schaefer, A. S. et al. A 3′ UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes Immun. 11, 45–54 (2010).
Ozturk, A., Famili, P. & Vieira, A. R. The antimicrobial peptide DEFB1 is associated with caries. J. Dent. Res. 89, 631–636 (2010).
Peyssonnaux, C. et al. Critical role of HIF-1α in keratinocyte defense against bacterial infection. J. Invest. Dermatol. 128, 1964–1968 (2008).
Steinbrech, D. S. et al. Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J. Surg. Res. 84, 127–133 (1999).
Tandara, A. A. & Mustoe, T. A. Oxygen in wound healing — more than a nutrient. World J. Surg. 28, 294–300 (2004).
Furuta, G. T. et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 (2001).
Louis, N. A. et al. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J. Cell Biochem. 99, 1616–1627 (2006).
Elson, D. A., Ryan, H. E., Snow, J. W., Johnson, R. & Arbeit, J. M. Coordinate up-regulation of hypoxia inducible factor (HIF)-1α and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 60, 6189–6195 (2000).
Albina, J. E. et al. HIF-1 expression in healing wounds: HIF-1α induction in primary inflammatory cells by TNF-α. Am. J. Physiol. Cell Physiol. 281, C1971–1977 (2001).
Shweiki, D., Itin, A., Soffer, D. & Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843–845 (1992).
Glover, L. E. et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl Acad. Sci. USA 110, 19820–19825 (2013).
Clavien, P. A., Petrowsky, H., DeOliveira, M. L. & Graf, R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 356, 1545–1559 (2007).
Pirenne, J. et al. Influence of ischemia-reperfusion injury on rejection after liver transplantation. Transplant. Proc. 29, 366–367 (1997).
Watt, K. D., Lyden, E. R., Gulizia, J. M. & McCashland, T. M. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 12, 134–139 (2006).
Schneider, M. et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology 138, 1143–1154 (2010).
Chouker, A. et al. In vivo hypoxic preconditioning protects from warm liver ischemia–reperfusion injury through the adenosine A2B receptor. Transplantation 94, 894–902 (2012).
Hart, M. L. et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology 135, 1739.e3–1750.e3 (2008).
Cheng, K. et al. Hypoxia-inducible factor-1α regulates β cell function in mouse and human islets. J. Clin. Invest. 120, 2171–2183 (2010).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Kebir, H. et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nature Med. 13, 1173–1175 (2007).
Dang, E. V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TReg cells. J. Exp. Med. 208, 1367–1376 (2011).
O'Connor, W. Jr et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunol. 10, 603–609 (2009).
Ben-Shoshan, J., Maysel-Auslender, S., Mor, A., Keren, G. & George, J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1α. Eur. J. Immunol. 38, 2412–2418 (2008).
Bartels, K., Karhausen, J., Clambey, E. T., Grenz, A. & Eltzschig, H. K. Perioperative organ injury. Anesthesiology 119, 1474–1489 (2013).
Park, S. W. et al. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J. Immunol. 189, 5421–5433 (2012).
Gelman, S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology 82, 1026–1060 (1995).
Schrier, R. W. & Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 351, 159–169 (2004).
Bove, T. et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: a prospective, double-blind, randomized clinical trial. Circulation 111, 3230–3235 (2005).
Kumar, A. B. & Suneja, M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology 114, 964–970 (2011).
Hill, P. et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 19, 39–46 (2008).
Grenz, A. et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 5, e137 (2008).
Kapitsinou, P. P. et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J. Clin. Invest. 124, 2396–2409 (2014). An important study that, for the first time, implicates kidney protection by HIF2α expressed in vascular endothelial cells.
Mole, D. R. et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg. Med. Chem. Lett. 13, 2677–2680 (2003).
Masson, N. & Ratcliffe, P. J. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J. Cell Sci. 116, 3041–3049 (2003).
Chan, D. A., Sutphin, P. D., Yen, S. E. & Giaccia, A. J. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1α. Mol. Cell. Biol. 25, 6415–6426 (2005).
Kong, D. et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 65, 9047–9055 (2005).
Wang, R., Zhou, S. & Li, S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr. Med. Chem. 18, 3168–3189 (2011).
Xia, Y., Choi, H. K. & Lee, K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 49, 24–40 (2012).
Ku, J. H. et al. Effect of dutasteride on the expression of hypoxia-inducible factor-1α, vascular endothelial growth factor and microvessel density in rat and human prostate tissue. Scand. J. Urol. Nephrol. 43, 445–453 (2009).
Puppo, M. et al. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1α and -2α. Mol. Cancer Ther. 7, 1974–1984 (2008).
Palayoor, S. T. et al. PX-478, an inhibitor of hypoxia-inducible factor-1α, enhances radiosensitivity of prostate carcinoma cells. Int. J. Cancer 123, 2430–2437 (2008).
Rey, S. et al. Synergistic effect of HIF-1α gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc. Natl Acad. Sci. USA 106, 20399–20404 (2009).
Aragones, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nature Genet. 40, 170–180 (2008). A study providing critically important insights into mechanisms of PHD-dependent tissue protection during ischaemia. Indeed, the authors found that PHD1 deletion and concomittant elevation in HIFs is associated with improved metabolic responses, thereby promoting hypoxia or ischaemia tolerance.
Rajagopalan, S. et al. Use of a constitutively active hypoxia-inducible factor-1α transgene as a therapeutic strategy in no-option critical limb ischemia patients: Phase I dose-escalation experience. Circulation 115, 1234–1243 (2007).
Creager, M. A. et al. Effect of hypoxia-inducible factor-1α gene therapy on walking performance in patients with intermittent claudication. Circulation 124, 1765–1773 (2011).
Bernhardt, W. M. et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J. Am. Soc. Nephrol. 21, 2151–2156 (2010). The first published clinical trial that successfully uses a PHD inhibitor in patients to achieve HIF stabilization and concomitant increases in erythropoitin production.
Flight, M. H. Deal watch: AstraZeneca bets on FibroGen's anaemia drug. Nature Rev. Drug Discov. 12, 730 (2013).
Macdougall, I. C. New anemia therapies: translating novel strategies from bench to bedside. Am. J. Kidney Dis. 59, 444–451 (2012).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Kaelin, W. G. Von Hippel–Lindau disease. Annu. Rev. Pathol. 2, 145–173 (2007). An important review paper that summarizes the discovery and functional role of the VHL gene in the post-translational regulation of HIF levels.
Fraisl, P., Mazzone, M., Schmidt, T. & Carmeliet, P. Regulation of angiogenesis by oxygen and metabolism. Dev. Cell 16, 167–179 (2009).
Liao, D. & Johnson, R. S. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 26, 281–290 (2007).
Pouyssegur, J., Dayan, F. & Mazure, N. M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 (2006).
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009).
Galluzzi, L., Kepp, O., Heiden, M. G. & Kroemer, G. Metabolic targets for cancer therapy. Nature Rev. Drug Discov. 12, 829–846 (2013).
Semenza, G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29, 625–634 (2009).
Asikainen, T. M. et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. 20, 1698–1700 (2006).
Hams, E. et al. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock 36, 295–302 (2011).
Percy, M. J. et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N. Engl. J. Med. 358, 162–168 (2008).
Simonson, T. S. et al. Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75 (2010).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Kastelein, J. J. et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 114, 1729–1735 (2006).
Takeda, N. et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24, 491–501 (2010).
Keith, B., Johnson, R. S. & Simon, M. C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nature Rev. Cancer 12, 9–22 (2012).
Branco-Price, C. et al. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell 21, 52–65 (2012).
Scholz, C. C. et al. Regulation of IL-1β-induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc. Natl Acad. Sci. USA 110, 18490–18495 (2013).
Cockman, M. E. et al. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc. Natl Acad. Sci. USA 103, 14767–14772 (2006).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Coleman, M. L. et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J. Biol. Chem. 282, 24027–24038 (2007).
Koditz, J. et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 110, 3610–3617 (2007).
Bartels, K., Grenz, A. & Eltzschig, H. K. Hypoxia and inflammation are two sides of the same coin. Proc. Natl Acad. Sci. USA 110, 18351–18352 (2013).
Riegel, A. K. et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 117, 2548–2555 (2011).
Idzko, M., Ferrari, D. & Eltzschig, H. K. Nucleotide signalling during inflammation. Nature 509, 310–317 (2014).
Koeppen, M., Eckle, T. & Eltzschig, H. K. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv. Pharmacol. 61, 145–186 (2011).
Aherne, C. M., Kewley, E. M. & Eltzschig, H. K. The resurgence of A2B adenosine receptor signaling. Biochim. Biophys. Acta 1808, 1329–1339 (2011).
Faigle, M., Seessle, J., Zug, S., El Kasmi, K. C. & Eltzschig, H. K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3, e2801 (2008).
Eltzschig, H. K. et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 99, 1100–1108 (2006).
Hart, M. L., Gorzolla, I. C., Schittenhelm, J., Robson, S. C. & Eltzschig, H. K. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 184, 4017–4024 (2010).
Eltzschig, H. K. et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113, 224–232 (2009).
Thompson, L. F. et al. Crucial role for ecto-5′- nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395–1405 (2004).
Ahmad, A. et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2α in pulmonary endothelial cells. Proc. Natl Acad. Sci. USA 106, 10684–10689 (2009).
Kong, T., Westerman, K. A., Faigle, M., Eltzschig, H. K. & Colgan, S. P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 20, 2242–2250 (2006).
Morote-Garcia, J. C., Rosenberger, P., Nivillac, N. M., Coe, I. R. & Eltzschig, H. K. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136, 607–618 (2009).
Eltzschig, H. K. et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 202, 1493–1505 (2005).
Casanello, P. et al. Equilibrative nucleoside transporter 1 expression is downregulated by hypoxia in human umbilical vein endothelium. Circ. Res. 97, 16–24 (2005).
Morote-Garcia, J. C., Rosenberger, P., Kuhlicke, J. & Eltzschig, H. K. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111, 5571–5580 (2008).
Chan, D. A., Sutphin, P. D., Denko, N. C. & Giaccia, A. J. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1α. J. Biol. Chem. 277, 40112–40117 (2002).
McDonough, M. A. et al. Selective inhibition of factor inhibiting hypoxia-inducible factor. J. Am. Chem. Soc. 127, 7680–7681 (2005).
Wang, G. L. & Semenza, G. L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 82, 3610–3615 (1993).
Knowles, H. J., Tian, Y. M., Mole, D. R. & Harris, A. L. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 95, 162–169 (2004).
Ivan, M. et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 99, 13459–13464 (2002).
Nangaku, M. et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler. Thromb. Vasc. Biol. 27, 2548–2554 (2007).
Zaman, K. et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, 21(waf1/cip1), and erythropoietin. J. Neurosci. 19, 9821–9830 (1999).
Warshakoon, N. C. et al. Design and synthesis of a series of novel pyrazolopyridines as HIF-1α prolyl hydroxylase inhibitors. Bioorg. Med. Chem. Lett. 16, 5687–5690 (2006).
Choi, S. M. et al. Clioquinol, a Cu(II)/Zn(II) chelator, inhibits both ubiquitination and asparagine hydroxylation of hypoxia-inducible factor-1α, leading to expression of vascular endothelial growth factor and erythropoietin in normoxic cells. J. Biol. Chem. 281, 34056–34063 (2006).
Siddiq, A. et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J. Biol. Chem. 280, 41732–41743 (2005).
Jiang, B. H. et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12, 363–369 (2001).
Majumder, P. K. et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1- dependent pathways. Nature Med. 10, 594–601 (2004).
Mie Lee, Y. et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1α activity. Biochem. Biophys. Res. Commun. 300, 241–246 (2003).
Qian, D. Z. et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1α. Cancer Res. 66, 8814–8821 (2006).
Kong, X. et al. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1α. Mol. Cell. Biol. 26, 2019–2028 (2006).
Hur, E. et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1α/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol. Pharmacol. 62, 975–982 (2002).
Osada, M., Imaoka, S. & Funae, Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1α protein. FEBS Lett. 575, 59–63 (2004).
Mabjeesh, N. J. et al. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 62, 2478–2482 (2002).
Isaacs, J. S. et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J. Biol. Chem. 277, 29936–29944 (2002).
Kaluz, S., Kaluzova, M. & Stanbridge, E. J. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1α C-terminal activation domain. Mol. Cell. Biol. 26, 5895–5907 (2006).
Yeo, E. J. et al. Amphotericin B blunts erythropoietin response to hypoxia by reinforcing FIH-mediated repression of HIF-1. Blood. 107, 916–923 (2006).
Kung, A. L. et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 6, 33–43 (2004).
Verma, R. P. & Hansch, C. Camptothecins: a SAR/QSAR study. Chem. Rev. 109, 213–235 (2009).
Rapisarda, A. et al. Schedule-dependent inhibition of hypoxia-inducible factor-1α protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 64, 6845–6848 (2004).
Acknowledgements
The authors acknowledge S. A. Eltzschig for artwork during the preparation of this manuscript. This work is supported by US National Institutes of Health grants (DK097075, HL092188, DK083385, HL098294, HL114457, DK50189, HL60569, DK95491 and HL119837) and by grants from the Crohn's and Colitis Foundation of America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Eltzschig, H., Bratton, D. & Colgan, S. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13, 852–869 (2014). https://doi.org/10.1038/nrd4422
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4422
This article is cited by
-
Role of pH-sensing receptors in colitis
Pflügers Archiv - European Journal of Physiology (2024)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Interplay of hypoxia-inducible factors and oxygen therapy in cardiovascular medicine
Nature Reviews Cardiology (2023)
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Hypoxia-Inducible Factor 1α and Its Role in Lung Injury: Adaptive or Maladaptive
Inflammation (2023)