Key Points
-
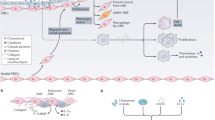
Atherosclerosis is the primary cause of major adverse cardiovascular events (MACE) — that is, coronary artery disease, heart attacks and stroke — and is thus the underlying pathology of the leading causes of death in the western world.
-
Traditionally, atherosclerosis was conceptualized as a progressive narrowing of artery lumen resulting from accumulation of cholesterol-rich plaque, which ultimately leads to plaque rupture, thrombosis and MACE. Accordingly, therapeutic efforts were directed at lowering blood cholesterol levels and addressing other cardiovascular risk factors.
-
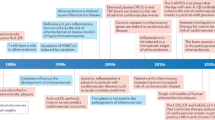
Although cardiovascular risk can be substantially lowered by hydroxy-3-methylglutaryl-CoA reductase inhibitors or statins, which reduce low-density lipoprotein cholesterol, or antihypertensives and antithrombotics, the residual risk of another MACE in these patients is estimated to be 70–80%.
-
It is becoming increasingly clear that inflammation within the arterial vessel wall and development of atherosclerotic plaques represent equally important and poorly treated contributors to MACE. Evidence now indicates that inflammation is a driver of cardiovascular mortality.
-
Several major clinical trials with statins — which have an anti-inflammatory action — have demonstrated that reducing inflammation has a major therapeutic benefit.
-
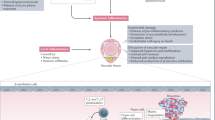
Several key anti-inflammatory targets have been identified and associated therapeutics are now being developed for the treatment of MACE — these include phospholipase A2 inhibitors, antileukotrienes and anticytokine therapeutics.
-
However, difficulties remain in assessing the potential of novel anti-inflammatory therapeutics to reduce MACE because of the high cost, duration and large numbers of patients required to conduct cardiovascular outcome clinical trials. In addition, there are currently no accepted surrogate end points for the reduction of MACE, although recent advances have been made in the use of biomarkers and imaging techniques.
Abstract
Atherosclerosis is the primary cause of heart disease and stroke and is thus the underlying pathology of the leading causes of death in the western world. Although risk can be reduced by lowering lipid levels, the equally important contribution of inflammation to the development of cardiovascular disease is not adequately addressed by existing therapies. Here, we summarize the evidence supporting a role for inflammation in the pathogenesis of atherosclerosis, discuss agents that are currently in the clinic and provide a perspective on the challenges faced in the development of drugs that target vascular inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
National Institutes of Health: National Heart, Lung and Blood Institute. 2009 NHLBI Morbidity and Mortality Chart Book. National Heart, Lung and Blood Institute [online], (2009).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000).
Glass, C. K. & Witztum, J. L. Atherosclerosis: the road ahead. Cell 104, 503–516 (2001).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Libby, P., Ridker, P., Göran, K. & Hansson, K. Inflammation in atherosclerosis: from pathophysiology to practice. JACC Cardiovasc. Imaging 54, 2129–2138 (2009). An excellent review of the role of inflammation in atherosclerosis.
Dreschler, M., Megens, R., van Zandvoort, M., Weber, C. & Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010).
Klingenberg, R. & Hansson, G. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur. Heart J. 30, 2838–2844 (2009).
Virmani, R., Kolodgie, F., Burke, A., Farb, A. & Schwartz, S. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000). This paper established clear morphological correlates with plaques that are ruptured leading to MACE.
O'Connor, R. et al. Part 9: acute coronary syndromes: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 122 (Suppl. 2), 422–465 (2010).
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004).
Mehta, S. et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358, 527–533 (2001).
Tardif, J.-C., Heinonen, T., Orloff, D. & Libby, P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation 113, 2936–2942 (2006). An excellent review of the current status of imaging and biomarker tools that are used to detect atherosclerotic disease.
Nissen, S. et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 292, 2217–2225 (2004).
Barter, P. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Kastelein, J. et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358, 1431–1443 (2008).
The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008).
Reifenberg, K. et al. Role of C-reactive protein in atherogenesis: can the apolipoprotein E knockout mouse provide the answer? Arterioscler. Thromb. Vasc. Biol. 25, 1641–1646 (2005).
Ridker, P. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005). This was the first detailed analysis demonstrating the relationship between lowering an inflammatory marker, hsCRP, and reducing MACE.
Nissen, S. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 (2005).
Zacho, J. et al. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 359, 1897–1908 (2008).
Ridker, P. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008). This was a landmark study demonstrating that statins reduced the incidence of MACE in patients with 'normal' LDL-C levels.
Schindhelm, R., vanDerZwan, L., Teerlink, T. & Scheffer, P. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 55, 1462–1470 (2009).
Blankenberg, S. et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 121, 2388–2397 (2010).
Möckel, M. et al. Role of N-terminal pro-B-type natriuretic peptide in risk stratification in patients presenting in the emergency room. Clin. Chem. 51, 1624–1631 (2005).
Wu, A. The role of cardiac troponin in the recent redefinition of acute myocardial infarction. Clin. Lab. Sci. 17, 50–52 (2004).
Fayad, Z. Cardiovascular molecular imaging. Arterioscler. Thromb. Vasc. Biol. 29, 981–981 (2009).
Tall, A., Yvan-Charvet, L., Terasaka, N., Pagler, T. & Wang, N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell. Metab. 7, 365–375 (2008).
Yvant-Charvet, L. et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30, 1430–1438 (2010).
Ridker, P. M., Rifai, N., Pfeffer, M. A., Sacks, F. & Braunwald, E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation 100, 230–235 (1999).
Ridker, P. M. et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 344, 1959–1965 (2001).
Tsou, C. L. et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909 (2007).
Yu, X. et al. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc. Natl Acad. Sci. USA 89, 6953–6957 (1992).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Zapolska-Downar, D., Siennicka, A., Kaczmarczyk, M., Kołodziej, B. & Naruszewicz, M. Simvastatin modulates TNFα-induced adhesion molecules expression in human endothelial cells. Life Sci. 75, 1287–1302 (2004).
Aikawa, M. et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 103, 276–283 (2001).
Steffens, S. & Mach, F. Anti-inflammatory properties of statins. Semin. Vasc. Med. 4, 417–422 (2004).
Schönbeck, U. & Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 109, II18–26 (2004).
Zhou, Q. & Liao, J. Pleiotropic effects of statins — basic research and clinical perspectives. Circ. J. 74, 818–826 (2010).
Tardif, J. et al. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 371, 1761–1768 (2008).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell. Metab. 6, 386–397 (2007).
Rosenson, R. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc. Drugs Ther. 23, 93–101 (2009).
Suckling, K. Phospholipase A2s: developing drug targets for atherosclerosis. Atherosclerosis 212, 357–366 (2010).
The Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 375, 1536–1544 (2010).
Wilensky, R. et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nature Med. 10, 1059–1066 (2008).
Serruys, P. et al. Effects of the direct lipoprotein-associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 118, 1172–1182 (2008). This research provided supporting evidence for a beneficial effect of Lp-PLA2 inhibition on coronary plaque formation.
Shaposhnik, Z., Wang, X., Trias, J., Fraser, H. & Lusis, A. The synergistic inhibition of atherogenesis in apoE−/− mice between pravastatin and the sPLA2 inhibitor varespladib (A-002). J. Lipid Res. 50, 623–629 (2009).
Hartford, M. et al. CRP, interleukin-6, secretory phospholipase A2 group IIA, and intercellular adhesion molecule-1 during the early phase of acute coronary syndromes and long-term follow-up. Int. J. Cardiol. 108, 55–62 (2006).
Rosenson, R., Elliott, M., Stasiv, Y., Hislop, C. & for the PLASMA II Investigators. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease. Eur. Heart J. 16 Nov 2010 (doi:10.1093/eurheartj/ehq374).
Peters-Golden, M. & Henderson, W. R. Jr. Leukotrienes. N. Engl. J. Med. 357, 1841–1854 (2007).
DeCaterina, R. & Zampolli, A. From asthma to atherosclerosis-5-lipoxygenase, leukotrienes, and inflammation. N. Engl. J. Med. 350, 4–7 (2004).
Bäck, M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 23, 41–48 (2009).
Lötzer, K., Funk, C. & Habenicht, A. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim. Biophys. Acta 1736, 30–37 (2005).
Funk, C. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nature Rev. Drug Discov. 4, 664–672 (2005).
Mehrabian, M. & Allayee, H. 5-lipoxygenase and atherosclerosis. Curr. Opin. Lipidol. 14, 447–457 (2003).
Dwyer, J. et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N. Engl. J. Med. 350, 29–37 (2004).
Zhao, L. et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nature Med. 10, 966–973 (2004).
Tabibiazar, R. et al. Signature patterns of gene expression in mouse atherosclerosis and their correlation to human coronary disease. Physiol. Genomics. 22, 213–226 (2005).
Spanbroek, R. et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc. Natl Acad. Sci. USA 100, 1238–1243 (2003).
Cipollone, F. et al. Association between 5-lipoxygenase expression and plaque instability in humans. Arterioscler. Thromb. Vasc. Biol. 25, 1665–1670 (2005).
Helgadottir, A. et al. Association between the gene encoding 5 lipoxygenase-activating protein and stroke replicated in a Scottish population. Am. J. Hum. Genet. 76, 505–509 (2005).
Helgadottir, A. et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nature Genet. 36, 233–239 (2004).
Assimes, T. et al. Common polymorphisms of ALOX5 and ALOX5AP and risk of coronary artery disease. Hum. Genet. 4, 399–408 (2008).
Hakonarson, H. et al. Effects of a 5 lipoxygenase activating protein inhibitor on biomarkers associated with risk of myocardial infarction. JAMA 293, 2245–2256 (2005).
Drazen, J. et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nature Genet. 22, 168–170 (1999).
Tardiff, J.-C. et al. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ. Cardiovasc. Imaging 3, 298–307 (2010). This research provided initial data linking a potent LT inhibitor with the reduction of coronary plaque as measured by coronary multi-detector computed tomography.
Nelken, N. A., Coughlin, S. R., Gordon, D. & Wilcox, J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J. Clin. Invest. 88, 1121–1127 (1991).
Cushing, S. D. et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl Acad. Sci. USA 87, 5134–5138 (1990).
Charo, I. F. et al. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc. Natl Acad. Sci. USA 91, 2752–2756 (1994).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998). This research demonstrated that CCR2 targets monocytes to developing plaques and promotes atherosclerosis in mice.
Zernecke, A., Shagdarsuren, E. & Weber, C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 28, 1897–1908 (2008).
Barlic, J., Zhang, Y., Foley, J. F. & Murphy, P. M. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor γ-dependent pathway. Circulation 114, 807–819 (2006).
Gilbert, J. et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum c-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 15 March 2011 (doi:10.10.1016/j.amjcard.2010.11.005). A small study that demonstrated that neutralization of CCR2 reduces inflammation in patients who are at high risk for MACE.
Aiello, R. et al. CCR2 receptor blockade alters blood monocyte subpopulations but does not affect atherosclerotic lesions in apoE−/− mice. Atherosclerosis 208, 370–375 (2010).
Piccinini, A. et al. Rationally evolving MCP-1/CCL2 into a decoy protein with potent anti-inflammatory activity in vivo. J. Biol. Chem. 285, 8782–8792 (2010).
Koenen, R. R. et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nature Med. 15, 97–103 (2009).
Serbina, N. & Pamer, E. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunol. 7, 311–317 (2006).
Saag, K. et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 59, 762–784 (2008).
Popa, C. et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 64, 303–305 (2005).
Jacobsson, L. et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 34, 1213–1218 (2005).
Ridker, P. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J. Thromb. Haemost. 7 (Suppl.1), 332–339 (2009).
Fearon, W. & Fearon, D. Inflammation and cardiovascular disease; role of the interleukin-1 receptor antagonist. Circulation. 117, 2577–2579 (2008).
Haverslag, R., Pasterkamp, G. & Hoefer, I. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc. Hematol. Disord. Drug Targets 8, 252–260 (2008).
Lindå, H. et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N. Engl. J. Med. 361, 1081–1087 (2009).
Crossman, D. et al. Investigation of the effect of Interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (the MRC-ILA-HEART Study). Trials 25, 8 (2008).
White, H. et al. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Athersclerotic plaque By Initiation of darapLadIb TherapY comparing darapladib versus placebo in patients with coronary heart disease. Am. Heart J. 160, 655–661 (2010).
Acknowledgements
The authors would like to thank D. Jones for his assistance in the preparation of this manuscript. This work was supported by the US National Institutes of Health grant R01 HL052773 (I.C.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Israel F. Charo is a member of the scientific advisory boards of ChemCentryx Inc. and VIA Pharmaceuticals, and a consultant for Bay City Capital.
Rebecca Taub is an employee, stockholder and officer of VIA Pharmaceuticals, which has an interest in developing therapeutics including leukotriene inhibitors to treat cardiovascular disease.
Related links
Glossary
- Low-density lipoprotein
-
(LDL). LDL is one of the five major groups of lipoproteins that enable lipids such as cholesterol and triglycerides to be transported within the bloodstream.
- Major adverse cardiovascular events
-
(MACE). A composite of cardiovascular events, typically including unstable angina, myocardial infarction, stroke and cardiovascular-related death.
- Myocardial infarction
-
(MI). An event caused by occlusion of coronary blood flow, typically resulting from the rupture of a coronary atherosclerotic plaque and subsequent thrombosis leading to damage to the heart muscle.
- Acute coronary syndrome
-
(ACS). Acute cardiovascular ischaemia resulting in unstable angina pectoris or myocardial infarction owing to impairment of blood flow within a coronary artery.
- ST-segment elevation
-
An ST-segment elevation refers to a finding on a 12-lead electrocardiogram, in which the trace in the ST-segment is abnormally high above the isoelectric line. The finding of ST-segment elevations in select leads is consistent with myocardial infarction.
- LDL cholesterol
-
(LDL-C). A measurement of the amount of cholesterol in the blood that is associated with low-density lipoprotein (LDL) particles. High LDL-C is associated with atherosclerotic cardiovascular disease.
- High density lipoprotein
-
(HDL). A dense lipoprotein that forms complexes with cholesterol, which is typically composed of a number of lipoproteins such as apolipoprotein A1, apolipoprotein A2 and other components. HDL transports cholesterol from the peripheral tissue in the body to the liver where it is secreted as biliary cholesterol or bile salts.
- Intima medial thickness
-
(IMT). The thickness of the intima within the carotid arteries as measured by an echocardiogram is correlated with the level of atherosclerotic disease.
- C-reactive protein
-
[CRP]. An acute phase protein that correlates with inflammatory disease processes, including atherosclerotic cardiovascular disease.
- High-sensitivity CRP
-
(hsCRP). An assay that was developed to more accurately measure levels of C-reactive protein (CRP) in the blood, particularly relatively low levels of CRP.
- Myeloperoxidase
-
(MPO). A protein that is expressed in the granules of most leukocytes, including neutrophils and monocytes. It is linked to inflammation and oxidative stress, and correlates with cardiovascular risk. MPO is released by leukocytes in a state of inflammation and it catalyses the formation of several reactive species. It has a role in the host defence against microorganisms.
- Troponin
-
A complex of three proteins (troponin T, troponin I and troponin C) that bind to the thin filament (actin) of striated muscle, which regulates muscle contraction. Following injury to heart muscle cells, the intact troponin complex, along with free troponin subunits, is released into the blood. The amino acid sequences for cardiac troponin T and troponin I isoforms are considerably different from their skeletal muscle counterparts and levels of troponin T can therefore be used to measure heart damage.
- Intravascular ultrasound
-
(IVUS). An invasive technique in which an ultrasound device is inserted into the coronary arteries via a catheterization procedure.
- Phospholipase A2
-
(PLA2). A series of phospholipases that hydrolyse the N2 ester bond of phospholipids to release fatty acid products and lysophospholipids.
- Lipoprotein-associated PLA2
-
(Lp-PLA2; also known as PAFAH). A phospholipase A2 (PLA2) enzyme that travels mainly with low-density lipoprotein (LDL). It is an enzyme that is produced by inflammatory cells and hydrolyses oxidized phospholipids in LDL.
- Secretory PLA2
-
A group of several secreted phospholipases, some of which are induced during inflammatory responses and/or are associated with oxidized low-density lipoprotein (LDL)-cholesterol particles. Proposed to be involved in the generation of atherogenic fatty acids.
- Leukotrienes
-
Inflammatory mediators that are produced mainly by white blood cells, macrophages and mast cells, and are composed of leukotriene B4 (LTB4) and cysteinyl leukotrienes LTC4, LTD4 and LTE4.
- Arachidonate 5-lipoxygenase
-
(5-LO).The rate limiting enzyme in the production of leukotrienes.
- 5-LO-activating protein
-
(FLAP). A protein that is responsible for activating the arachidonate 5-lipoxygenase (5-LO) enzyme within the leukotriene pathway.
- Chemokine CC motif ligand 2
-
(CCL2; also known as MCP1). A peptide that binds to the chemokine CC motif receptor 2 (CCR2) and is important in monocyte migration and inflammation.
- Chemokine receptor 2
-
A chemokine receptor for chemokine CC motif ligand 2 (CCL2), which is a chemokine that specifically mediates monocyte chemotaxis and inflammation.
Rights and permissions
About this article
Cite this article
Charo, I., Taub, R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov 10, 365–376 (2011). https://doi.org/10.1038/nrd3444
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3444
This article is cited by
-
Systems immunology-based drug repurposing framework to target inflammation in atherosclerosis
Nature Cardiovascular Research (2023)
-
CHOP Increases TRIB3-Dependent miR-208 Expression to Potentiate Vascular Smooth Muscle Cell Proliferation and Migration by Downregulating TIMP3 in Atherosclerosis
Cardiovascular Drugs and Therapy (2022)
-
Anti-inflammatory Action of Statins in Cardiovascular Disease: the Role of Inflammasome and Toll-Like Receptor Pathways
Clinical Reviews in Allergy & Immunology (2021)
-
Hypothetical Pathway for Formation of Cholesterol Microcrystals Initiating the Atherosclerotic Process
Cell Biochemistry and Biophysics (2020)
-
Interactive effects of interferon-gamma functional single nucleotid polymorphism (+874 T/A) with cardiovascular risk factors in coronary heart disease and early myocardial infarction risk
Molecular Biology Reports (2020)