Key Points
-
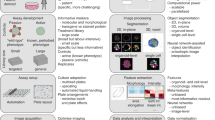
Modern cell-imaging techniques have enabled the development of more biologically relevant cell-based assays for all therapeutic areas that can be used throughout drug discovery R&D. The ability to automate and multiplex imaging technologies to increase throughput has further expanded the capabilities of cellular imaging technology as a tool for drug discovery.
-
Cellular imaging is defined as the use of a system or technology capable of visualizing a cell population, single cell or subcellular structures that is applied in combination with image-analysis tools. These systems extract a two-dimensional pixel array of information (a digital image) from a particular biological event or cell type.
-
In target identification and validation, techniques based on fluorescence, such as fluorescence energy transfer (FRET) and fluorescence lifetime imaging (FLIM), can be used to study the dynamics and localization of protein targets within living cells. Flow cytometry is another imaging technique that can be used at this stage in drug discovery for identifying target antigens for the development of antibody-based therapeutics. Automated microscopy has also made it possible to look at phenotypic changes of entire cell populations and study the effect of drugs on various cell processes to identify drug targets.
-
Fluorometric imaging plate readers (FLIPR) have been used for several years in industry for compound screening. New versions of this technology offer improved resolution and integrated data-analysis systems, and the use of embryonic stem cells as an alternative to primary or transformed cells is beginning to show potential.
-
Parallel efficacy and toxicity testing is another application of cellular imaging that is beginning to show promise, by enabling the simultaneous assessment of desired on-target effects alongside off-target toxic effects. Advances in genotoxicity testing such as the image-based micronucleus assay are being adopted as alternatives to conventional genotoxicity tests and require less test compound.
-
Finally, cellular imaging is likely to be key to the discovery and use of biomarkers for monitoring drug activity and cell fate in vivo, with a view to better characterizing drugs and improving understanding of their mechanism of action.
Abstract
Traditional screening paradigms often focus on single targets. To facilitate drug discovery in the more complex physiological environment of a cell or organism, powerful cellular imaging systems have been developed. The emergence of these detection technologies allows the quantitative analysis of cellular events and visualization of relevant cellular phenotypes. Cellular imaging facilitates the integration of complex biology into the screening process, and addresses both high-content and high-throughput needs. This review describes how cellular imaging technologies contribute to the drug discovery process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hood, L. & Perlmutter, R. M. The impact of systems approaches on biological problems in drug discovery. Nature Biotechnol. 22, 1215–1217 (2004).
Sams-Dodd, F. Target-based drug discovery: is something wrong? Drug Discov. Today 10, 139–147 (2005).
Besson, D., Yeow, K., Lang, P. & Scheer, A. HTS and cellular biology at Serono. Curr. Drug Discov. 29–32 (2003).
Comley, J. High content screening: emerging importance of novel reagents/probes and pathway analysis. Drug Discov. World 6, 31–54 (2005).
Ramm, P. Image-based screening: a technology in transition. Curr. Opin. Biotechnol. 16, 41–48 (2005).This review describes the advantages and disadvantages of using cellular imaging technologies in screening, and provides clues of what future cellular imaging systems requirements are for HTS purposes.
Bivona, T. G. & Philips, M. R. Analysis of Ras and Rap activation in living cells using fluorescent Ras binding domains. Methods 37, 138–145 (2005).
Voss, T. C., Demarco, I. A. & Day, R. N. Quantitative imaging of protein interactions in the cell nucleus. Biotechniques 38, 413–424 (2005).
Sekar, R. B. & Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 160, 629–633 (2003).
Errington, R. J. et al. Advanced microscopy solutions for monitoring the kinetics and dynamics of drug–DNA targeting in living cells. Adv. Drug Deliv. Rev. 57, 153–167 (2005).
von Arnim, C. A. et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J. Biol. Chem. 280, 17777–17785 (2005).
Herzenberg, L. A. et al. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin. Chem. 48, 1819–1827 (2002).
Geuijen, C. A. et al. Affinity ranking of antibodies using flow cytometry: application in antibody phage display-based target discovery. J. Immunol. Methods 302, 68–77 (2005).
Florian, S. et al. Detection of molecular targets on the surface of CD34+/CD38– stem cells in various myeloid malignancies. Leuk. Lymphoma 47, 207–222 (2006).
Heinemann, A. et al. Basophil responses to chemokines are regulated by both sequential and cooperative receptor signaling. J. Immunol. 165, 7224–7233 (2000).
Tanaka, M. et al. An unbiased cell morphology-based screen for new, biologically active small molecules. PLoS Biol. 3, e128 (2005).
Miller, S. C. & Mitchison, T. J. Synthesis and phenotypic screening of a guanine-mimetic library. Chembiochem. 5, 1010–1012 (2004).
Yarrow, J. C., Feng, Y., Perlman, Z. E., Kirchhausen, T. & Mitchison, T. J. Phenotypic screening of small molecule libraries by high throughput cell imaging. Comb. Chem. High Throughput. Screen. 6, 279–286 (2003).Highlights the power of cellular imaging in finding active small molecules and shows how compound progression can be carried out to find the molecular target affecting cellular phenotype.
Yarrow, J. C., Totsukawa, G., Charras, G. T. & Mitchison, T. J. Screening for cell migration inhibitors via automated microscopy reveals a Rho-kinase inhibitor. Chem. Biol. 12, 385–395 (2005).
Ramm, P. et al. Automated screening of neurite outgrowth. J. Biomol. Screen. 8, 7–18 (2003).
Richards, G. R., Millard, R. M., Leveridge, M., Kerby, J. & Simpson, P. B. Quantitative assays of chemotaxis and chemokinesis for human neural cells. Assay. Drug Dev. Technol. 2, 465–472 (2004).
Bahnson, A. et al. Automated measurement of cell motility and proliferation. BMC Cell Biol. 6, 19 (2005).
Berns, K. et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004).
Eggert, U. S. et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2, e379 (2004).
Mattheakis, L. C. et al. Optical coding of mammalian cells using semiconductor quantum dots. Anal. Biochem. 327, 200–208 (2004).
Edwards, B. S., Oprea, T., Prossnitz, E. R. & Sklar, L. A. Flow cytometry for high-throughput, high-content screening. Curr. Opin. Chem. Biol. 8, 392–398 (2004).
Irish, J. M. et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118, 217–228 (2004).An impressive paper demonstrating how flow cytometry using antiphospho antibodies can provide new ways of clustering cancer-patient populations according to signalling pathways.
Morgan, E. et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 110, 252–266 (2004).
Wong, C. K., Cheung, P. F., Ip, W. K. & Lam, C. W. Interleukin-25-induced chemokines and interleukin-6 release from eosinophils is mediated by p38 mitogen-activated protein kinase, c-Jun N-terminal kinase, and nuclear factor-κB. Am. J. Respir. Cell Mol. Biol. 33, 186–194 (2005).
Rausch, O. Use of high-content analysis for compound screening and target selection. IDrugs 8, 573–577 (2005).
Ramm, P. Imaging systems in assay screening. Drug Discov. Today 4, 401–410 (1999).
Wu, C. C., Reilly, J. F., Young, W. G., Morrison, J. H. & Bloom, F. E. High-throughput morphometric analysis of individual neurons. Cereb. Cortex 14, 543–554 (2004).
Burnett, P. et al. Fluorescence imaging of electrically stimulated cells. J. Biomol. Screen. 8, 660–667 (2003).
Ramm, P. Advanced image analysis systems in cell, molecular and neurobiology applications. J. Neurosci. Methods 54, 131–149 (1994).
Takahashi, Y., Sawada, R., Ishibashi, K., Mikuni, S. & Kinjo, M. Analysis of cellular functions by multipoint fluorescence correlation spectroscopy. Curr. Pharm. Biotechnol. 6, 159–165 (2005).
Wouters, F. S., Verveer, P. J. & Bastiaens, P. I. Imaging biochemistry inside cells. Trends Cell Biol. 11, 203–211 (2001).
Watson, P., Jones, A. T. & Stephens, D. J. Intracellular trafficking pathways and drug delivery: fluorescence imaging of living and fixed cells. Adv. Drug Deliv. Rev. 57, 43–61 (2005).
Gasparri, F., Mariani, M., Sola, F. & Galvani, A. Quantification of the proliferation index of human dermal fibroblast cultures with the ArrayScan high-content screening reader. J. Biomol. Screen. 9, 232–243 (2004).
Schroeder, K. S. & Neagle, B. D. FLIPR: a new instrument for accurate, high throughput optical screening. J. Biomol. Screen. 1, 75–80 (1996).Describes the first application of a fluorometric imaging plate reader (FLIPR), one of the most widely adopted cellular imaging tools in the pharmaceutical industry so far.
Reynen, P. H., Martin, G. R., Eglen, R. M. & MacLennan, S. J. Characterization of human recombinant α2A-adrenoceptors expressed in Chinese hamster lung cells using intracellular Ca2+ changes: evidence for cross-talk between recombinant α2A- and native α1-adrenoceptors. Br. J. Pharmacol. 129, 1339–1346 (2000).
Nickolls, S. A., Fleck, B., Hoare, S. R. & Maki, R. A. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J. Pharmacol. Exp. Ther. 313, 1281–1288 (2005).
Gopalakrishnan, S. M. et al. An offline-addition format for identifying GPCR modulators by screening 384-well mixed compounds in the FLIPR. J. Biomol. Screen. 10, 46–55 (2005).
Patel, K. et al. Activity of diadenosine polyphosphates at P2Y receptors stably expressed in 1321N1 cells. Eur. J. Pharmacol. 430, 203–210 (2001).
Benjamin, E. R. et al. State-dependent compound inhibition of Nav1.2 sodium channels using the FLIPR Vm dye: on-target and off-target effects of diverse pharma-cological agents. J. Biomol. Screen. 11, 29–39 (2005).
Benjamin, E. R. et al. Validation of a fluorescent imaging plate reader membrane potential assay for high-throughput screening of glycine transporter modulators. J. Biomol. Screen. 10, 365–373 (2005).
Giuliano, K. A. & Taylor, D. L. Fluorescent-protein biosensors: new tools for drug discovery. Trends Biotechnol. 16, 135–140 (1998).
Ghosh, R. N., Grove, L. & Lapets, O. A quantitative cell-based high-content screening assay for the epidermal growth factor receptor-specific activation of mitogen-activated protein kinase. Assay. Drug Dev. Technol. 2, 473–481 (2004).
Kapur, R. Fluorescence imaging and engineered biosensors: functional and activity-based sensing using high content screening. Ann. NY Acad. Sci. 961, 196–197 (2002).
Grepin, C. et al. Increasing the quality of compounds isolated during primary screening: high content screening with Acumen Explorer. Curr. Drug Discov. 3, 37–42 (2003).
Jager, S. et al. A modular, fully integrated ultra-high-throughput screening system based on confocal fluorescence analysis techniques. J. Biomol. Screen. 8, 648–659 (2003).
Oakley, R. H. et al. The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay. Drug Dev. Technol. 1, 21–30 (2002).
Fowler, A., Davies, I. & Norey, C. A multi-modality assay platform for ultra-high throughput screening. Curr. Pharm. Biotechnol. 1, 265–281 (2000).
Almholt, D. L. et al. Nuclear export inhibitors and kinase inhibitors identified using a MAPK-activated protein kinase 2 redistribution screen. Assay. Drug Dev. Technol. 2, 7–20 (2004).
Almholt, K. et al. Changes in intracellular cAMP reported by a redistribution assay using a cAMP-dependent protein kinase-green fluorescent protein chimera. Cell Signal. 16, 907–920 (2004).
Bertelsen, M. & Sanfridson, A. Inflammatory pathway analysis using a high content screening platform. Assay. Drug Dev. Technol. 3, 261–271 (2005).Describes the application of cellular imaging to compound profiling by monitoring its efficacy across various signalling pathways.
Li, Z. et al. Identification of gap junction blockers using automated fluorescence microscopy imaging. J. Biomol. Screen. 8, 489–499 (2003).
Lundholt, B. K. et al. Identification of Akt pathway inhibitors using redistribution screening on the FLIPR and the IN Cell 3000 analyzer. J. Biomol. Screen. 10, 20–29 (2005).Describes the use of a HT cellular imaging device to screen compounds and a high-content screening cellular imaging platform to understand the mode of action of a compound.
Borchert, K. M. et al. High-content screening assay for activators of the Wnt/Fzd pathway in primary human cells. Assay. Drug Dev. Technol. 3, 133–141 (2005).
Horrocks, C., Halse, R., Suzuki, R. & Shepherd, P. R. Human cell systems for drug discovery. Curr. Opin. Drug Discov. Devel. 6, 570–575 (2003).
Obinata, M. Possible applications of conditionally immortalized tissue cell lines with differentiation functions. Biochem. Biophys. Res. Commun. 286, 667–672 (2001).
McNeish, J. Embryonic stem cells in drug discovery. Nature Rev. Drug Discov. 3, 70–80 (2004).This review highlights the potential power of using stem cells in target discovery and primary screening.
Allen, M. et al. Deficiency of the stress kinase p38α results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191, 859–870 (2000).
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 4391–4396 (2002).
Trounson, A. The production and directed differentiation of human embryonic stem cells. Endocr. Rev. 24 Jan 2006 [epub ahead of print].
Laschinski, G., Vogel, R. & Spielmann, H. Cytotoxicity test using blastocyst-derived euploid embryonal stem cells: a new approach to in vitro teratogenesis screening. Reprod. Toxicol. 5, 57–64 (1991).
Mitchell, K. E. et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells 21, 50–60 (2003).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
Ulrich, R. & Friend, S. H. Toxicogenomics and drug discovery: will new technologies help us produce better drugs? Nature Rev. Drug Discov. 1, 84–88 (2002).
Smith, D. A. & van de, W. H. Pharmacokinetics and metabolism in early drug discovery. Curr. Opin. Chem. Biol. 3, 373–378 (1999).
Waters, M. D. & Fostel, J. M. Toxicogenomics and systems toxicology: aims and prospects. Nature Rev. Genet. 5, 936–948 (2004).
Riley, R. J. & Kenna, J. G. Cellular models for ADMET predictions and evaluation of drug–drug interactions. Curr. Opin. Drug Discov. Dev. 7, 86–99 (2004).
Nersesyan, K., Melikyan, G. S. & Stopper, H. Genotoxic activity of newly synthesized derivatives of cyano-pyridone in murine cells in vivo and in vitro. Tsitol. Genet. 38, 44–48 (2004).
Tice, R. R. et al. Report from the working group on the in vivo mammalian bone marrow chromosomal aberration test. Mutat. Res. 312, 305–312 (1994).
Fenech, M. In vitro micronucleus technique to predict chemosensitivity. Methods Mol. Med. 111, 3–32 (2005).
Fenech, M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ. Health Perspect. 101 (Suppl. 3), 101–107 (1993).
Ekins, S., Nikolsky, Y. & Nikolskaya, T. Techniques: application of systems biology to absorption, distribution, metabolism, excretion and toxicity. Trends Pharmacol. Sci. 26, 202–209 (2005).
Pritchard, J. F. et al. Making better drugs: decision gates in non-clinical drug development. Nature Rev. Drug Discov. 2, 542–553 (2003).
Lesko, L. J. & Atkinson, A. J. Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 41, 347–366 (2001).
Frank, R. & Hargreaves, R. Clinical biomarkers in drug discovery and development. Nature Rev. Drug Discov. 2, 566–580 (2003).
De Meyer, G. & Shapiro, F. Biomarker development:the road to clinical utility. Curr. Drug Discov. 12, 23–37 (2003).
Rolan, P., Atkinson, A. J. Jr & Lesko, L. J. Use of biomarkers from drug discovery through clinical practice: report of the Ninth European Federation of Pharmaceutical Sciences Conference on Optimizing Drug Development. Clin. Pharmacol. Ther. 73, 284–291 (2003).
Koop, R. Combinatorial biomarkers: from early toxicology assays to patient population profiling. Drug Discov. Today 10, 781–788 (2005).
Nishimura, T. et al. Disease proteomics toward bedside reality. J. Gastroenterol. 40 (Suppl. 16), 7–13 (2005).
Liu, E. T. Expression genomics and drug development: towards predictive pharmacology. Brief. Funct. Genomic. Proteomic. 3, 303–321 (2005).
Shibazaki, M., Takeuchi, T., Ahmed, S. & Kikuchia, H. Blockade by SB203580 of Cyp1a1 induction by 2,3, 7,8-tetrachlorodibenzo-p-dioxin, and the possible mechanism: possible involvement of the p38 mitogen-activated protein kinase pathway in shuttling of Ah receptor overexpressed in COS-7 cells. Ann. NY Acad. Sci. 1030, 275–281 (2004).
Traxler, P. et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 64, 4931–4941 (2004).
Koga, H. et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 45, 1622–1630 (2005).
Bick, R. J. et al. Fluorescence imaging microscopy of cellular markers in ischemic vs non-ischemic cardiomyopathy after left ventricular unloading. J. Heart Lung Transplant. 24, 454–461 (2005).
Kelloff, G. J. & Sigman, C. C. New science-based endpoints to accelerate oncology drug development. Eur. J. Cancer 41, 491–501 (2005).
Swanson, B. N. Delivery of high-quality biomarker assays. Dis. Markers 18, 47–56 (2002).
Colburn, W. A. Biomarkers in drug discovery and development: from target identification through drug marketing. J. Clin. Pharmacol. 43, 329–341 (2003).
Rathbun, R. C. Surrogate markers for assessing treatment response in HIV disease. Ann. Pharmacother. 27, 450–455 (1993).Demonstrates how cellular imaging can be used in the field of clinical biomarkers by monitoring CD4 cell count in patients with HIV.
Perlman, Z. E. et al. Multidimensional drug profiling by automated microscopy. Science 306, 1194–1198 (2004).A study that demonstrates the power of cell systems tools when combined with cellular imaging to profile drugs and understand their mechanism of action.
Nolan, J. P., Lauer, S., Prossnitz, E. R. & Sklar, L. A. Flow cytometry: a versatile tool for all phases of drug discovery. Drug Discov. Today 4, 173–180 (1999).
Asadullah, K., Sterry, W. & Volk, H. D. Analysis of cytokine expression in dermatology. Arch. Dermatol. 138, 1189–1196 (2002).
Duramad, P., McMahon, C. W., Hubbard, A., Eskenazi, B. & Holland, N. T. Flow cytometric detection of intracellular TH1/TH2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 13, 1452–1458 (2004).
de Weck, A. L. et al. Lymphocyte proliferation, lymphokine production, and lymphocyte receptors in ageing and various clinical conditions. Springer Semin. Immunopathol. 7, 273–289 (1984).
Lacombe, F. & Belloc, F. Flow cytometry study of cell cycle, apoptosis and drug resistance in acute leukemia. Hematol. Cell Ther. 38, 495–504 (1996).
Krutzik, P. O., Irish, J. M., Nolan, G. P. & Perez, O. D. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin. Immunol. 110, 206–221 (2004).
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Mater. 4, 435–446 (2005).
Rubart, M. Two-photon microscopy of cells and tissue. Circ. Res. 95, 1154–1166 (2004).
Paris, S. & Sesboue, R. Metastasis models: the green fluorescent revolution? Carcinogenesis 25, 2285–2292 (2004).
Chishima, T. et al. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 57, 2042–2047 (1997).
Kan, Z. & Liu, T. J. Video microscopy of tumor metastasis: using the green fluorescent protein (GFP) gene as a cancer-cell-labeling system. Clin. Exp. Metastasis 17, 49–55 (1999).
Rice, B. W., Cable, M. D. & Nelson, M. B. In vivo imaging of light-emitting probes. J. Biomed. Opt. 6, 432–440 (2001).
Uhrbom, L., Nerio, E. & Holland, E. C. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nature Med. 10, 1257–1260 (2004).
Kumar, S., Kahn, M. A., Dinh, L. & de Vellis, J. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J. Neurosci. Res. 54, 754–765 (1998).
Gao, X. et al. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 16, 63–72 (2005).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).Illustrates the potential of quantum dot beads to track cell fate in vivo , and exemplifies the possibility of multiplexing cellular imaging technology.
Fischer, H. P. Towards quantitative biology: integration of biological information to elucidate disease pathways and to guide drug discovery. Biotechnol. Annu. Rev. 11, 1–68 (2005).
Butcher, E. C. Can cell systems biology rescue drug discovery? Nature Rev. Drug Discov. 4, 461–467 (2005).
Sachs, K., Perez, O., Pe'er, D., Lauffenburger, D. A. & Nolan, G. P. Causal protein-signaling networks derived from multiparameter single-cell data. Science 308, 523–529 (2005).
Miyawaki, A., Sawano, A. & Kogure, T. Lighting up cells: labelling proteins with fluorophores. Nature Cell Biol. (Suppl.), S1–S7 (2003).
Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. Creating new fluorescent probes for cell biology. Nature Rev. Mol. Cell Biol. 3, 906–918 (2002).
Verkhusha, V. V. & Lukyanov, K. A. The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nature Biotechnol. 22, 289–296 (2004).
Lukyanov, K. A., Chudakov, D. M., Lukyanov, S. & Verkhusha, V. V. Innovation: photoactivatable fluorescent proteins. Nature Rev. Mol. Cell Biol. 6, 885–891 (2005).
Hercend, T. et al. Immunotherapy with lymphokine-activated natural killer cells and recombinant interleukin-2: a feasibility trial in metastatic renal cell carcinoma. J. Biol. Response Mod. 9, 546–555 (1990).
Nagy, R. D. et al. Stem cell transplantation as a therapeutic approach to organ failure. J. Surg. Res. 129, 152–160 (2005).
Nir, T. & Dor, Y. How to make pancreatic β cells — prospects for cell therapy in diabetes. Curr. Opin. Biotechnol. 16, 524–529 (2005).
Keller, G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 (2005).
Wollert, K. C. et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 (2004).
Tang, Y. L. Cellular therapy with autologous skeletal myoblasts for ischemic heart disease and heart failure. Methods Mol. Med. 112, 193–204 (2005).
Sykes, M. & Nikolic, B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature 435, 620–627 (2005).
Radbruch, A. & Thiel, A. Cell therapy for autoimmune diseases: does it have a future? Ann. Rheum. Dis. 63 (Suppl. 2), ii96–ii101 (2004).
Mattson, M. P. Emerging neuroprotective strategies for Alzheimer's disease: dietary restriction, telomerase activation, and stem cell therapy. Exp. Gerontol. 35, 489–502 (2000).
Tuszynski, M. H. et al. A Phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Med. 11, 551–555 (2005).
Brundin, P. et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain 123, 1380–1390 (2000).
Widner, H. et al. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N. Engl. J. Med. 327, 1556–1563 (1992).
Bulte, J. W. Hot spot MRI emerges from the back-ground. Nature Biotechnol. 23, 945–946 (2005).
Eriksson, P. S. et al. Neurogenesis in the adult human hippocampus. Nature Med. 4, 1313–1317 (1998).
Zhu, J., Wu, X. & Zhang, H. L. Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr. Drug Targets 6, 97–110 (2005).
Frangioni, J. V. & Hajjar, R. J. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation 110, 3378–3383 (2004).
Vogt, A. et al. Cell-active dual specificity phosphatase inhibitors identified by high-content screening. Chem. Biol. 10, 733–742 (2003).
DiMasi, J. A., Hansen, R. W. & Grabowski, H. G. The price of innovation: new estimates of drug development costs. J. Health Econ. 22, 151–185 (2003).
Acknowledgements
The authors would like to thank C. Waltzinger for her expertise in flow cytometry and C. Johnson-Leger, D. Besson and D. Perrin for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Charge-coupled device camera
-
A charge-coupled device is a silicon chip whose surface is divided into light-sensitive pixels. When a photon hits a pixel it registers as an electric charge that can be counted. With large pixel arrays and high sensitivity, CCDs can create high-resolution images and are often incorporated into cameras to take such pictures.
- Laser-scanning microscopy
-
A laser beam passing a light source aperture is focused into a small focal volume in a fluorescent specimen. The mixture of emitted fluorescent and reflected laser light from the illuminated spot is then separated so that the laser light passes through and reflects the fluorescent light on to the detection apparatus.
- Fluorescence resonance energy transfer
-
(FRET). FRET is a distance-dependent interaction between the electronically excited states of two dyes that can be used to measure biological phenomena that produce changes in molecular proximity.
- Multiphoton microscopy
-
A standard technique for three-dimensional imaging of thick fluorescent specimens.
- Epifluorescence microscopy
-
This method uses a type of microscope that has a very bright light source. Ultraviolet, blue, yellow or red light from the light source is then used to energize the specimen, which will then re-emit light at various wavelengths to be viewed by the observer.
- Fluorescence-activated cell sorter
-
(FACS). A FACS is a machine that can rapidly separate cells in suspension on the basis of size and the colour of their fluorescence.
- TUNEL
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labelling is used for quantifying apoptosis at the single-cell-level based on labelling of free 3′-OH terminals that occur as a result of DNA strand breaks.
- Intravital microscopy
-
The observation of cell fate in a living organism by the detection of green fluorescent protein.
Rights and permissions
About this article
Cite this article
Lang, P., Yeow, K., Nichols, A. et al. Cellular imaging in drug discovery. Nat Rev Drug Discov 5, 343–356 (2006). https://doi.org/10.1038/nrd2008
Issue Date:
DOI: https://doi.org/10.1038/nrd2008
This article is cited by
-
A deep learning dataset for sample preparation artefacts detection in multispectral high-content microscopy
Scientific Data (2024)
-
Fast detection of slender bodies in high density microscopy data
Communications Biology (2023)
-
Single object profiles regression analysis (SOPRA): a novel method for analyzing high-content cell-based screens
BMC Bioinformatics (2022)
-
A robust unsupervised machine-learning method to quantify the morphological heterogeneity of cells and nuclei
Nature Protocols (2021)
-
Arrayed functional genetic screenings in pluripotency reprogramming and differentiation
Stem Cell Research & Therapy (2019)