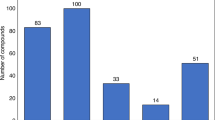
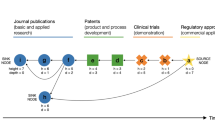
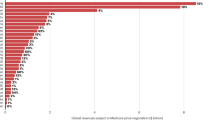
Abstract
Long-term declines in the productivity of pharmaceutical R&D threaten the sustainability of the current business model. A major driver of the decline has been the rising cost, and increased rate of failure, of projects across the industry, caused in part by the confluence of new approaches, novel technologies, higher go/no-go hurdles and organizational challenges. Here we examine several of the top culprits that are charged with driving this decline and the merits of the cases against them, and speculate on the emerging trends that will underpin the industry's future productivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grabowski, H. G. Public policy and pharmaceutical innovation. Health Care Financ. Rev. 4, 75–87 (1982).
Fruits of Genomics (Lehman Brothers, New York, 2001).
Edmunds, R. et al. Splicing a cost squeeze into the genomics revolution. McKinsey Quarterly 2, 15–18 (2001).
Hopkins, A. & Groom, C. The druggable genome. Nature Rev. Drug Discov. 1, 727–730 (2002).
Ma, P. & Zemmel, R. Value of novelty? Nature Rev. Drug Discov. 1, 571–572 (2002).
Booth, B. & Zemmel, R. Quest for the best. Nature Rev. Drug Discov. 2, 838–841 (2003).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Wenlock, M. C., Austin, R. P., Barton, P., Davis, A. M. & Leeson, P. D. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem. 46, 1250–1256 (2003).
Vieth, M. et al. Characteristic physical properties and structural fragments of marketed oral drugs. J. Med. Chem. 47, 224–232 (2004).
Sneader, W. Drug Prototypes and their Exploitation (John Wiley & Sons, Chichester, 1996).
Ostholm, I. Drug Discovery: A Pharmacist's Story (Swedish Pharmaceutical, Stockholm, 1995).
Horrobin, D. F. Innovation in pharmaceuticals. J. R. Soc. Med. 93, 341–345 (2000).
Horrobin, D. F. Modern biomedical research: an internally self-consistent universe with little contact with medical reality. Nature Rev. Drug Discov. 2, 151–154 (2003).
Erickson, D. Wanted: drug hunters. In Vivo 45, (2003).
Reichert, J. M. & Paquette, C. Therapeutic recombinant proteins: trends in US approvals 1982 to 2002. Curr. Opin. Mol. Ther. 5, 139–147 (2003).
Acknowledgements
We would like to thank several McKinsey colleagues for their support and hard work in helping pull together the analyses in this review, including J. Hong, D. Lennon, P. Ma and E. McCafferty.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Booth, B., Zemmel, R. Prospects for productivity. Nat Rev Drug Discov 3, 451–456 (2004). https://doi.org/10.1038/nrd1384
Issue Date:
DOI: https://doi.org/10.1038/nrd1384
This article is cited by
-
In silico drug repositioning using deep learning and comprehensive similarity measures
BMC Bioinformatics (2021)
-
Strategies to identify candidate repurposable drugs: COVID-19 treatment as a case example
Translational Psychiatry (2021)
-
Precision medicine review: rare driver mutations and their biophysical classification
Biophysical Reviews (2019)