Key Points
-
Presently available drugs are ineffective in controlling clinical pain in most patients and abolish the pain in only few. It is suggested that this failure arises from the fact that these drugs were developed to target neurons, rather than spinal cord glia (astrocytes and microglia).
-
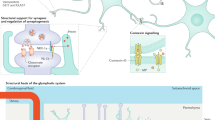
Until recently, glia were thought of simply as housekeepers for neurons, regulating the extracellular ionic environment and removing debris. Recently, it has become recognized that these glia dynamically modulate the function of neurons under both physiological and pathological conditions.
-
Glial activation has been demonstrated to be both necessary and sufficient for enhanced nociception (pain transmission) in every animal model tested to date.
-
Activated glia enhance pain via the release of a variety of neuroactive substances. Central to these effects is glial release of pro-inflammatory cytokines (tumor-necrosis factor, interleukin-1 (IL-1) and IL-6). These, in turn, activate a cascade of events leading to the release of a host of neuroexcitatory substances (such as nitric oxide, prostaglandins, growth factors, excitatory amino acids and so on).
-
In animal models, the following block/reverse changes in nociception in every animal model of clinical pain studied to date: disruption of glial activation (fluorocitrate and minocycline); pro-inflammatory cytokine antagonists (Kineret, Remicade, Enbrel); pro-inflammatory cytokine synthesis inhibitors (propentofylline, thalidomide); and disrupting pro-inflammatory cytokine signalling and synthesis (leflunomide, methotrexate, p38 MAP kinase, IL-10).
-
These studies strongly indicate that drug discovery should seriously consider targeting spinal cord glial activation as a broad-spectrum solution to presently unresolved clinical pain syndromes.
Abstract
In many clinical pain syndromes, painful sensations are greatly amplified so that normally innocuous sensations, such as light touch or warmth, are perceived as pain. Presently available drugs are ineffective in controlling such pain in most patients and abolish the pain in only few. Why do they fail? These drugs were developed to target neurons that transmit nociceptive ('pain') information. However, glia have recently been recognized as powerful modulators of nociception, and could hold the key to the control of clinical pain and present a new target for drug discovery. This review examines the evidence for glial regulation of nociception and pharmacological approaches that might successfully control glially driven clinical pain syndromes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McQuay, H., Carroll, D., Jadad, A. R., Wiffen, P. & Moore, A. Anticonvulsant drugs for management of pain: a systematic review. Brit. Med. J. 311, 1047–1052 (1995).
McQuay, H. J. et al. A systematic review of antidepressants in neuropathic pain. Pain 68, 217–227 (1996).
Watkins, L. R. & Maier, S. F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 82, 981–1011 (2002). This article reviews the immunology of peripheral nerves, dorsal root ganglia and spinal nerves; the evidence from animal models of immune involvement in pathological pain; and the evidence that diverse human clinical pain syndromes involve an immune component.
Woolf, C. J. & Salter, M. W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000). An excellent review of neuronal changes implicated in creation and maintenance of exaggerated pain states.
Pekny, M. in Progress in Brain Research: Glial Cell Funtion (eds Castellano-Lopez, B. & Nieto-Sampedro, M.) 23–30 (Elsevier, Amsterdam, 2001).
Benveniste, E. N. in Neuroglia (eds Kettenmann, H. & Ransom, B. R.) 700–716 (Oxford, New York, 1995).
Perry, V. H. Macrophages and the Nervous System (Landes, Austin, 1994).
Gehrmann, J. & Kreutzberg, G. W. in Neuroglia (eds Kettenmann, H. & Ransom, B. R.) 883–904 (Oxford, New York, 1995).
Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215 (1999). Synapses can no longer be considered as simply a presynaptic neuron and a postsynaptic neuron. Rather, three entities are involved, the third being astrocytes. A review of the evidence that astrocytes 'listen' to neuronal communication and 'talk back' to the neurons is provided.
Garrison, C. J., Dougherty, P. M., Kajander, K. C. & Carlton, S. M. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 565, 1–7 (1991). This research article is historically important as it provides the first evidence that nerve damage, which creates neuropathic pain, also activates spinal cord glial
Garrison, C. J., Dougherty, P. M. & Carlton, S. M. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp. Neurol. 129, 237–243 (1994). Historically important, this article provides the first evidence that drugs that inhibit neuropathic pain also inhibit glial activation. It provided the first evidence that, at minimum, neuropathic pain and glial activation are strongly correlated.
Watkins, L. R., Milligan, E. D. & Maier, S. F. Glial activation: a driving force for pathological pain. Trends Neurosci. 24, 450–455 (2001). Evidence is reviewed that spinal cord glia are key mediators in the creation and maintenance of exaggerated pain states.
Berg-Johnsen, J., Paulsen, R. E., Fonnum, F. & Langmoen, I. A. Changes in evoked potentials and amino acid content during fluorocitrate action studied in rat hippocampal cortex. Exp. Brain Res. 96, 241–246 (1993).
Hassel, B., Paulsen, R. E., Johnson, A. & Fonnum, F. Selective inhibition of glial cell metabolism by fluorocitrate. Brain Res. 249, 120–124 (1992).
Tikka, T. M. & Koistinaho, J. E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 166, 7527–7533 (2001).
Meller, S. T., Dykstra, C., Grzbycki, D., Murphy, S. & Gebhart, G. F. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 33, 1471–1478 (1994). Provides the first evidence that disrupting glial activation blocks exaggerated pain responses. In addition, it is the first evidence that activation of glia, in their role as immune cells, is sufficient to induce exaggerated pain responses.
Watkins, L. R., Martin, D., Ulrich, P., Tracey, K. J. & Maier, S. F. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 71, 225–235 (1997).
Milligan, E. D. et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 861, 105–116 (2000).
Milligan, E. D. et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain. J. Neurosci. 23, 1026–1040 (2003).
Raghavendra, V., Tanga, F. & DeLeo, J. A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 306, 624–630 (2003). Anatomical and pharmacological evidence supports the intriguing hypothesis that microglia are key in the initiation of exaggerated pain states, but that astrocytes (and not microglia) are crucial for the maintenance of enhanced pain.
Ledeboer, A. et al. Selective inhibition of spinal cord microglial activation attenuates mechanical allodynia in rat models of pathological pain. Proc. Soc. Neurosci. (in the press).
Cholewinski, A. J., Hanley, M. R. & Wilkin, G. P. A phosphoinositide-linked peptide response in astrocytes: evidence for regional heterogeneity. Neurochem. Res. 13, 389–394 (1988).
Beaujouan, J. C. et al. Marked regional heterogeneity of 125I-Bolton Hunter substance P binding and substance P-induced activation of phospholipase C in astrocyte cultures from the embryonic or newborn rat. J. Neurochem. 54, 669–675 (1990).
Sung, B., Lim, G. & Mao, J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 23, 2899–2910 (2003).
Ochalski, P. A., Frankenstein, U. N., Hertzberg, E. L. & Nagy, J. I. Connexin-43 in rat spinal cord: localization in astrocytes and identification of heterotypic astro-oligodendrocytic gap junctions. Neurosci. 76, 931–945 (1997).
Li, W. E. & Nagy, I. Activation of fibres in rat sciatic nerve alters phosphorylation state of connexin-43 at astrocytic gap junctions in spinal cord: evidence for junction regulation by neuronal–glial interactions. Neurosci. 97, 113–123 (2000).
Palma, C. et al. Functional characterization of substance P receptors on cultured human spinal cord astrocytes: synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia 21, 183–193 (1997).
Tikka, T., Fiebich, B. L., Goldsteins, G., Keinanen, R. & Koistinaho, J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 21, 2580–2588 (2001).
Bartlett, P. F. Pluripotential hemopoietic stem cells in adult mouse brain. Proc. Natl Acad. Sci. USA 79, 2722–2725 (1982).
Carson, M. J., Reilly, C. R., Sutcliffe, J. G. & Lo, D. Mature microglia resemble immature antigen-presenting cells. Glia 22, 72–85 (1998).
Fedoroff, S. in Neuroglia (eds Kettenmann, H. & Ransom, B. R.) 162–184 (Oxford, New York, 1995).
Lee, J. C., Mayer-Proschel, M. & Rao, M. S. Gliogenesis in the central nervous system. Glia 30, 105–121 (2000).
Watkins, L. R., Hansen, M. K., Nguyen, K. T., Lee, J. E. & Maier, S. F. Dynamic regulation of the proinflammatory cytokine, interleukin-1β: molecular biology for non-molecular biologists. Life Sci. 65, 449–481 (1999).
Milligan, E. D. et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 21, 2808–2819 (2001). The first demonstration that activation of spinal cord glia, in their role as immune cells; (i) is sufficient to induce thermal hyperalgesia and mechanical allodynia, (ii) induces the production and release of pro-inflammatory cytokines, and (iii) this pro-inflammatory cytokine release is causal to the resultant pain enhancement.
Harrison, J. K. et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl Acad. Sci. USA 95, 10896–10901 (1998).
Watkins, L. R., Milligan, E. D. & Maier, S. F. in Advances in Pain Research and Therapy (eds Dostrovsky, J. O., Carr, D. B. & Koltzenberg, M.) 369–385 (IASP, Seattle, 2003).
Verge, G. et al. Mapping fractalkine and its receptor (CX3CR1) in a rat model of sciatic inflammatory neuropathy (SIN). Proc. Soc. Neurosci. 28, 455.3 (2002).
Chapman, G. A. et al. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J. Neurosci. 20, RC87 (1–5) (2000).
Dinarello, C. A. in Cytokines and Pain (eds Watkins, L. R. & Maier, S. F.) 1–19 (Birkhauser, Basel, 1999). An excellent review of pro-inflammatory cytokine molecular biology and cellular signalling, with a focus on their role in pain facilitation.
Maier, S. F. & Watkins, L. R. Cytokines for psychologists: implications of bi-directional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 105, 83–107 (1998). This review article examines the role of pro-inflammatory cytokines in a wide array of peripheral, brain and spinal cord processes. It is aimed at non-specialists to introduce them to this research area
DeLeo, J. A., Colburn, R. W., Nochols, M. & Malhotra, A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J. Interferon Cytokine Res. 16, 695–700 (1996).
Falchi, M., Ferrara, G., Gharib, C. & Dib, B. Hyperalgesic effect of intrathecally administered interleukin-1 in rats. Drugs Exp. Clin. Res. 27, 97–101 (2001).
Reeve, A. J., Patel, S., Fox, A., Walker, K. & Urban, L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain 4, 247–257 (2000).
Tadano, T. et al. Induction of nociceptive responses by intrathecal injection of interleukin-1 in mice. Life Sci. 65, 255–261 (1999).
Oka, T. & Hori, T. in Cytokines and Pain (eds Watkins, L. R. & Maier, S. F.) 183–204 (Birkhauser, Basel, 1999).
Sweitzer, S. M., Martin, D. & DeLeo, J. A. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neurosci. 103, 529–539 (2001).
Clark, A. R., Dean, J. L. E. & Saklatvala, J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 546, 37–44 (2003).
Svensson, C. I. et al. Activation of p38 MAP kinase in spinal microglia is a critical link in inflammation induced spinal pain processing. J. Neurochem. 86, 1534–1544 (2003).
Svensson, C. I., Hua, X. Y., Protter, A. A., Powell, H. C. & Yaksh, T. L. Spinal p38 MAP kinase is necessary for NMDA induced spinal PGE2 release and thermal hyperalgesia. Neuroreport 14, 1153–1157 (2003).
Schafers, M., Svensson, C. I., Sommer, C. & Sorkin, L. S. Tumor necrosis factor induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 23, 2517–2521 (2003).
Jones, T. L. et al. Involvement of p38α MAPK in capsaicin-induced hyperalgesia. Proc. Soc. Neurosci. 28, 56.5 (2002).
Milligan, E. D. et al. Systemic administration of CNI-1493, a p38 MAP kinase inhibitor, blocks HIV-1 gp120-induced enhanced pain states in rats. J. Pain 2, 326–333 (2001).
Sweitzer, S. M., Schubert, P. & DeLeo, J. A. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J. Pharmacol. Exp. Ther. 297, 1210–1217 (2001).
Winkelstein, B. A., Rutkowski, M. D., Sweitzer, S. M., Pahl, J. L. & DeLeo, J. A. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J. Comp. Neurol. 2, 127–139 (2001).
Hashizume, H., Rutkowski, M. D., Weinstein, J. N. & DeLeo, J. A. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain 87, 159–169 (2000).
Sommer, C., Marziniak, M. & Myers, R. R. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain 74, 83–91 (1998).
George, A., Marziniak, M., Schafers, M., Toyka, K. V. & Sommer, C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-α, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain 88, 267–275 (2000).
Eriksson, T., Bjorkman, S. & Hoglund, P. Clinical pharmacology of thalidomide. Eur. J. Clin. Pharmacol. 5, 365–376 (2001).
Clemmensen, O. J., Olsen, P. Z. & Andersen, K. E. Thalidomide neurotoxicity. Arch. Dermatol. 120, 338–341 (1984).
Strle, K. et al. Interleukin-10 in the brain. Crit. Rev. Immunol. 21, 427–449 (2001).
Moore, K. W., deWaal Malefyt, R., Coffman, R. L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Ledeboer, A. et al. Regional and temporal expression patterns of interleukin-10, interleukin-10 receptor and adhesion molecules in the rat spinal cord during chronic relapsing EAE. J. Neuroimmunol. 136, 94–103 (2003).
Yu, C. -G., Fairbanks, C. A., Wilcox, G. L. & Yezierski, R. P. Effects of agmatine, interleukin-10 and cyclosporin on spontaneous pain behavior following excitotoxic spinal cord injury in rats. J. Pain 4, 129–140 (2003).
Laughlin, T. M., Bethea, J. R., Yezierski, R. P. & Wilcox, G. L. Cytokine involvement in dynorphin-induced allodynia. Pain 84, 159–167 (2000).
Plunkett, J. A., Yu, C. -G., Easton, J. M., Bethea, J. R. & Yezierski, R. P. Effects of interelukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exper. Neurol. 168, 144–154 (2001).
Hornfeldt, C. S. & Larson, A. A. Seizures induced by fluoroacetic acid and fluorocitric acid may involve chelation of divalent cations in the spinal cord. Eur. J. Pharmacol. 179, 307–313 (1990).
Yrjanheikki, J., Keinanen, R., Pellikka, M., Hokfelt, T. & Korstinaho, J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Neurobiology 95, 15769–15774 (1998).
Lin, S. et al. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition of p38 MAP kinase in rat cerebellar granule neurons. Neurosci. Lett. 315, 61–64 (2001).
Kary, S. & Burmester, G. R. Anakinra: the first interleukin-1 inhibitor in the treatment of rheumatoid arthritis. Int. J. Clin. Pract. 57 (2003).
Calabrese, L. H. Molecular differences in anticytokine therapies. Clin. Exp. Rheumatol. 21, 241–248 (2003).
Mielke, R. et al. Propentofylline in the treatment of vascular dementia and Alzheimer-type dementia: overview of phase I and phase II clinical trials. Alzheimer Dis. Assoc. Disord. 12, S29–35 (1998).
Rother, M. et al. Propentofylline in the treatment of Alzheimer's disease and vascular dementia: a review of phase III trials. Dement. Geriatr. Cogn. Disord. 9, 36–43 (1998).
Plaschke, K. et al. Neuromodulatory effect of propentofylline on rat brain under acute and long-term hypoperfusion. Br. J. Pharmacol. 133, 107–116 (2001).
Wu, Y. P., McRae, A., Rudolphi, K. & Ling, E. A. Propentofylline attenuates microglial reaction in the rat spinal cord induced by middle cerebral artery occlusion. Neurosci. Lett. 260, 17–20 (1999).
Numagami, Y., Marro, P. J., Mishra, O. P. & Delivoria-Papadopoulos, M. Effect of propentofylline on free radical generation during cerebral hypoxia in the newborn piglet. Neuroscience 84, 1127–1133 (1998).
Schubert, P. et al. Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann. NY Acad. Sci. 903, 24–33 (2000).
Schubert, P., Ogata, T., Marchini, C., Ferroni, S. & Rudolphi, K. Protective mechanisms of adenosine in neurons and glial cells. Ann. NY Acad. Sci. 825, 1–10 (1997).
Sawynok, J. & Liu, X. J. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog. Neurobiol. 69, 313–340 (2003).
Si, Q., Nakamura, Y., Ogata, T., Kataoka, K. & Schubert, P. Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res. 812, 97–104 (1998).
Matthews, S. J. & McCoy, C. Thalidomide: a review of approved and investigational uses. Clin. Ther. 25, 342–395 (2003).
Mujagic, H., Chabner, B. A. & Mujagic, Z. Mechanisms of action and potential therapeutic uses of thalidomide. Croat. Med. J. 43, 274–285 (2002).
Majumdar, S., Lamothe, B. & Aggarwal, B. B. Thalidomide suppresses NF-κB activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J. Immunol. 168, 2644–2651 (2002).
Gallily, R., Kipper-Galperin, M. & Brenner, T. Mycoplasma fermentans-induced inflammatory response of astrocytes: selective modulation by aminoguanidine, thalidomide, pentoxifylline and IL-10. Inflammation 23, 495–505 (1999).
Peterson, P. K. et al. Thalidomide inhibits tumor necrosis factor-α production by lipopolysaccharide- and lipoarabinomannan-stimulated human microglial cells. J. Infect. Dis. 172, 1137–1140 (1995).
Moreira, A. L. et al. Thalidomide exerts its inhibitory action on tumor necrosis factor-α by enhancing mRNA degradation. J. Exp. Med. 177, 1675–1680 (1993).
Corral, L. G. et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J. Immunol. 163, 380–386 (1999).
Li, J., Luo, S., Hong, W., Zhou, Z. & Zou, W. Influence of thalidomide on interleukin-6 and its transmission in multiple myeloma patients. Zhonghua Zhong Liu Za Zhi 24, 254–256 (2002).
Bauditz, J., Wedel, S. & Lochs, H. Thalidomide reduces tumour necrosis factor-α and interleukin-12 production in patients with chronic active Crohn's disease. Gut 50, 196–200 (2002).
Jin, S. H., Kim, T. I., Han, D. S., Shin, S. K. & Kim, W. H. Thalidomide suppresses the interleukin 1β-induced NF-κB signaling pathway in colon cancer cells. Ann. NY Acad. Sci. 973, 414–418 (2002).
Thiele, A. et al. Cytokine modulation and suppression of liver injury by a novel analogue of thalidomide. Eur. J. Pharmacol. 453, 325–334 (2002).
Rajkumar, S. V., Fonseca, R. & Witzig, T. E. Complete resolution of reflex sympathetic dystrophy with thalidomide treatment. Arch. Intern. Med. 161, 2502–2503 (2001).
Prager, J., Fleischman, J. & Lingua, G. Open label clinical experience of thalidomide in the treatment of complex regional pain syndrome Type 1. J. Pain 4, S68 (2003).
Sanders, S. & Harisdangkul, V. Leflunomide for the treatment of rheumatoid arthritis and autoimmunity. Am. J. Med. Sci. 323, 190–193 (2002).
Furst, D. E. Innovative treatment approaches for rheumatoid arthritis. Cyclosporin, leflunomide and nitrogen mustard. Baillieres Clin. Rheumatol. 9, 711–729 (1995).
Kraan, M. C. et al. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 43, 1820–1830 (2000).
Elkayam, O. et al. Active leflunomide metabolite inhibits interleukin-1β, tumour necrosis factor-α, nitric oxide, and metalloproteinase-3 production in activated human synovial tissue cultures. Ann. Rheum. Dis. 62, 440–443 (2003).
Cutolo, M. et al. Anti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 297–302 (2003).
Manna, S. K., Mukhopadhyay, A. & Aggarwal, B. B. Leflunomide suppresses TNF-induced cellular responses: effects on NF-κB, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J. Immunol. 165, 5962–5969 (2000).
Fox, R. I. et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin. Immunol. 93, 198–208 (1999).
Herrmann, M. L., Schleyerbach, R. & Kirschbaum, B. J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47, 273–289 (2000).
Miljkovic, D. et al. Leflunomide inhibits activation of inducible nitric oxide synthase in rat astrocytes. Brain Res. 889, 331–338 (2001).
Bao, L. et al. Adjuvant-induced arthritis: IL-1β, IL-6 and TNF-α are up-regulated in the spinal cord. Neuroreport 12, 3905–3908 (2001).
Bruce-Gregorios, J. H., Soucy, D. M., Chen, M. G. & Norenberg, M. D. Effect of methotrexate on glial fibrillary acidic protein content of astrocytes in primary culture. J. Neuropathol. Exp. Neurol. 50, 118–125 (1991).
el-Badawi, M. G., Fatani, J. A., Bahakim, H. & Abdalla, M. A. Light and electron microscopic observations on the cerebellum of guinea pigs following low-dose methotrexate. Exp. Mol. Pathol. 53, 211–222 (1990).
Longo-Sorbello, G. S. & Bertino, J. R. Current understanding of metrotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica 86, 121–127 (2001).
Chan, E. S. & Cronstein, B. N. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 4, 266–273 (2002).
Cronstein, B. N., Naime, D. & Ostad, E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv. Exp. Med. Biol. 370, 411–416 (1994).
Montesinos, M. C. et al. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 43, 656–663 (2000).
Neurath, M. F. et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin. Exp. Immunol. 115, 42–55 (1999).
Hayem, G., Domarle, O., Thuong-Guyot, M., Pocidalo, J. J. & Meyer, O. Effects of methotrexate on the oxidative metabolism of cultured rabbit articular chondrocytes. J. Rheumatol. 27, 1117–1120 (2000).
Rudwaleit, M. et al. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor-α, increase of interleukin-10, and predicted by the initial concentration of interleukin-4. Ann. Rheum. Dis. 59, 311–314 (2000).
Brody, M., Bohm, I. & Bauer, R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin-1β to the interleukin-1 receptor on target cells. Eur. J. Clin. Chem. Clin. Biochem. 31, 667–674 (1993).
Segal, R., Mozes, E., Yaron, M. & Tartakovsky, B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis Rheum. 32, 370–377 (1989).
Segal, R., Yaron, M. & Tartakovsky, B. Rescue of interleukin-1 activity by leucovorin following inhibition by methotrexate in a murine in vitro system. Arthritis Rheum. 33, 1745–1748 (1990).
Gregorios, J. B. et al. Morphologic alterations in rat brain following systemic and intraventricular methotrexate injection: light and electron microscopic studies. J. Neuropathol. Exp. Neurol. 48, 33–47 (1989).
Hommes, D. et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology 122, 7–14 (2002).
Lali, F. V., Hunt, A. E., Turner, S. J. & Foxwell, B. M. The pyridinyl imidazole inhibitor SF203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 275, 7395–7402 (2000).
Dunn, S. L., Young, E. A., Hall, M. D. & McNulty, S. Activation of astrocyte intracellular signaling pathways by interleukin-1 in rat primary striatal cultures. Glia 37, 31–42 (2002).
Jeohn, G. H. et al. Go6976 inhibits LPS-induced microglial TNF-α release by suppressing p38 MAP kinase activation. Neuroscience 114, 689–697 (2002).
Jin, S. X., Zhaung, Z. Y., Woolf, C. J. & Ji, R. R. p38 MAPK is activated after a spinal nerve ligation first in spinal cord microglia and then in DRG neurons and contributes to the generation of neuropathic pain. J. Neurosci. 23, 4017–4022 (2003).
Kim, S. Y. et al. Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport 13, 2483–2486 (2002).
Hamilton, T. A., Ohmori, Y., Tebo, J. M. & Kishore, R. Regulation of macropahge gene expression by pro- and anti-inflammatory cytokines. Pathobiology 67, 241–244 (1999).
Kontoyiannis, D. et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20, 3760–3770 (2001).
Donnelly, R. P., Dickensheets, H. & Finbloom, D. S. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19, 563–573 (1999).
Sawada, M., Suzumura, A., Hosoya, H., Marunouchi, T. & Nagatsu, T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 72, 1466–1471 (1999).
Foey, A. D. et al. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-α: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160, 920–928 (1998).
Huber, T. S. et al. Anticytokine therapies for acute inflammation and the systemic inflammatory response syndrome: IL-10 and ischemia/reperfusion injury. Shock 13, 425–434 (2000).
Bachis, A. et al. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J. Neurosci. 21, 3104–3112 (2001).
Milligan, E. D. et al. Inflammatory and chronic constriction injury-induced pain states are controlled by spinal delivery of viral and non-viral vectors encoding the anti-inflammatory gene, interleukin-10 (IL10). Proc. Soc. Neurosci. 29 (in the press).
Kastin, A. J., Akerstrom, V. & Pan, W. Interleukin-10 as a CNS therapeutic: the obstacle of the blood–brain/blood–spinal cord barrier. Brain Res. Mol. Brain Res. 114, 168–171 (2003).
Davidson, B. L. & Breakefield, X. O. Viral vectors for gene delivery to the nervous system. Nature Rev. Neurosci. 4, 353–364 (2003). This review article focuses on the promise of gene therapy for the resolution of a variety of clinical targets.
Xu, Z. L., Mizuguchi, H., Mayumi, T. & Hayakawa, T. Regulated gene expression from adenovirus vectors: a systematic comparison of various inducible systems. Gene 309, 145–151 (2003).
Wiesler-Frank, J., Milligan, E. D., Maier, S. F. & Watkins, L. R. in Encyclopedic Reference of Pam (eds Schmidt, R. & Willis, W. D.) (Springer, Berlin, in the press).
Volterra, A. & Bezzi, P. in The Tripartite Synapse (eds Volterra, A., Magistretti, P. J. & Hayden, P. G.) 164–182 (Oxford Univ. Press, Oxford, 2002).
Acknowledgements
Our laboratory is supported by grants from the National Institute on Drug Abuse, National Institute of Neurological Diseases and Stroke, and National Institute of Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Linda Watkins has received a grant from Avigen for work related to IL-10 gene therapy.
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
Glossary
- PAIN FACILITATION
-
Perceiving pain out of proportion to the initiating stimulus. Includes hyperalgesia and allodynia, below.
- HYPERALGESIA
-
Perceiving stimuli as more painful than they would normally be.
- ALLODYNIA
-
Perceiving normally non-painful stimuli as painful.
- PHENOTYPE SHIFT
-
As used here, means that a neuron changes what neurotransmitter it produces and releases, creating a functional change in the pain transmission system.
- GLIAL FIBRILLARY ACIDIC PROTEIN
-
(GFAP). An astrocyte-specific protein. Increases in GFAP are frequently used as a marker of astrocyte activation.
- p38 MAP KINASE
-
An intracellular signalling cascade, activated in response to pro-inflammatory cytokine receptor binding. Activation of this cascade leads to production of pro-inflammatory cytokines.
- IMMUNOCOMPETENT
-
Functioning similar to immune cells; that is, expressing receptors for bacteria/viruses and releasing pro-inflammatory cytokines and other immune cell products on activation.
- NEUROPATHIC PAIN
-
Allodynia/hyperalgesia caused by inflammation of and/or trauma to peripheral nerves.
- IL-6-NEUTRALIZING ANTIBODIES
-
Antibodies that bind to IL-6 and so prevent IL-6 from binding to its receptor.
- PERI-SPINAL INJECTION
-
Administering a drug into the cerebrospinal fluid surrounding the spinal cord; also called 'intrathecal'.
- SYNOVIAL TISSUES
-
Tissues encapsulating joints.
- NUCLEAR FACTOR-κB
-
(NF-κB). A transcription factor that is constitutively expressed. Its activation leads (among other effects) to the production of pro-inflammatory cytokines. Although constitutively expressed, its ability to move to the nucleus to bind to DNA is tonically inhibited by binding of IκB.
- INHIBITOR OF κB
-
(IκB). An inhibitor of NF-κB activation. It is binding to NF-κB that keeps this transcription factor from being able to move to the nucleus to activate messenger RNA transcription.
Rights and permissions
About this article
Cite this article
Watkins, L., Maier, S. GLIA: A novel drug discovery target for clinical pain. Nat Rev Drug Discov 2, 973–985 (2003). https://doi.org/10.1038/nrd1251
Issue Date:
DOI: https://doi.org/10.1038/nrd1251
This article is cited by
-
Andrographolide inhibits the activation of spinal microglia and ameliorates mechanical allodynia
Metabolic Brain Disease (2023)
-
Wnt3a/YTHDF1 Regulated Oxaliplatin-Induced Neuropathic Pain Via TNF-α/IL-18 Expression in the Spinal Cord
Cellular and Molecular Neurobiology (2023)
-
Behavioral and inflammatory sex differences revealed by celecoxib nanotherapeutic treatment of peripheral neuroinflammation
Scientific Reports (2022)
-
Spinal cord astrocytes regulate myocardial ischemia–reperfusion injury
Basic Research in Cardiology (2022)
-
Effects of high-frequency near infrared laser irradiation on experimental tooth movement–induced pain in rats
Lasers in Medical Science (2022)