Key Points
-
HIV-1 infection is characterized by a chronic phase during which the virus persists as integrated provirus in the genome of latently infected CD4+ T cells.
-
Cells latently infected with HIV-1 are not commonly recognized by the immune system owing to the absence of virus antigen expression on their cellular membrane and selection of escape mutants by natural infection-induced immune responses.
-
HIV-1 neutralizing antibodies are capable of reducing the level of virus replication and decreasing the pool of latently infected cells.
-
Antibodies with broadly neutralizing activity have been exploited to generate bispecific and trispecific molecules with multiple anti-HIV-1 envelope (HIV-1 Env) specificities to augment the breadth of recognition of diverse HIV-1 isolates.
-
Bispecific and trispecific molecules, such as dual-affinity re-targeting (DART) proteins, have been generated that combine broadly reactive anti-Env and anti-CD3 specificities to recruit cytotoxic T cells to eliminate latently infected cells that express HIV-1 Env upon provirus reactivation.
-
Preclinical and clinical studies are planned to evaluate the safety and activity of antibody-derived therapeutics (as single agents or combinations) that may be co-administered with agents capable of reversing HIV-1 latency.
Abstract
HIV-1 is a retrovirus that integrates into host chromatin and can remain transcriptionally quiescent in a pool of immune cells. This characteristic enables HIV-1 to evade both host immune responses and antiretroviral drugs, leading to persistent infection. Upon reactivation of proviral gene expression, HIV-1 envelope (HIV-1 Env) glycoproteins are expressed on the cell surface, transforming latently infected cells into targets for HIV-1 Env-specific monoclonal antibodies (mAbs), which can engage immune effector cells to kill productively infected CD4+ T cells and thus limit the spread of progeny virus. Recent innovations in antibody engineering have resulted in novel immunotherapeutics such as bispecific dual-affinity re-targeting (DART) molecules and other bi- and trispecific antibody designs that can recognize HIV-1 Env and recruit cytotoxic effector cells to kill CD4+ T cells latently infected with HIV-1. Here, we review these immunotherapies, which are designed with the goal of curing HIV-1 infection.
Similar content being viewed by others
Main
Efforts to develop new strategies to eradicate the latent pool of HIV-1-infected CD4+ T cells were ignited by the unexpected cure of the 'Berlin patient' by a bone marrow transplant1. In the setting of bone marrow ablation for leukaemia, the patient received bone marrow from a donor with a deletion in the HIV-1 receptor CC-chemokine receptor 5 (CCR5Δ32), which is resistant to CCR5-tropic HIV-1 (R5 HIV-1)1. Further interest in the possibility of a HIV-1 cure was stimulated by the case of the 'Mississippi baby', who received combination antiretroviral therapy (cART) immediately after birth2. Although the virus ultimately rebounded in this baby3, the initial cART prevented the reappearance of viraemia for more than a year2 and these clinical observations stimulated new efforts to develop novel strategies to cure AIDS.
During acute HIV-1 infection, CD8+ T cell immune responses are generated to eliminate virus-infected cells, leading to the partial control of virus replication that contributes to the virus set point4 (Fig. 1a). However, full control of virus replication is usually not achieved, even in individuals classified as elite controllers. Ultrasensitive assays that can detect plasma viraemia at levels below 50 copies per ml have demonstrated that the majority of individuals previously defined as elite controllers have blips of detectable viraemia of >1 copy per ml over time5,6. Nonetheless, elite controllers can limit the size of the latent pool; these individuals have 1.5 log lower median of infectious units per million (IUPM) cells compared with chronic HIV-1 progressors who do not naturally control virus replication7.
a | The blue line represents a representative estimate of the viraemia (virus load, copies per ml of plasma) in a HIV-1-infected patient. Initial HIV-1 replication within the CD4+ T cell compartment results in viraemia levels that can reach millions of copies per ml. Natural immune responses generally cannot control this level of virus replication. Upon initiation of combination antiretroviral therapy (cART), plasma levels of virus decline in three phases (indicated by 1, 2 and 3), which is related to the elimination of different subsets of infected cells172,173,174,175. This decline culminates in residual levels of virus in plasma that are below the limit of detection. Nonetheless, latently infected cells persist in patients maintained on cART even when virus load is undetectable18,19,20,21. Latency reversing agents (LRAs) can be used to reactivate the provirus41,77 while cART therapy is maintained according to the 'shock and kill' strategy77,176. Hypothetically, this strategy could eliminate the pool of latently infected cells, but clinical trail data have yet to provide evidence that this could be the final outcome60,76,77,78,79,80,81,82,82,83,84. b | This schematic represents the temporal impact that immune responses and drug therapy have on the changes in the level of virus load as displayed in part a. The diagnosis of HIV-1 infection and initiation of cART usually occurs at a time when immune responses are initiated but not yet effective in controlling virus replication as indicated in part a. This inefficient immune response results in the development of a pool of latently infected cells and cART cannot prevent their formation or eradicate them. The only opportunity for cART to be effective would be at a time when spontaneous virus replication takes place. In this scenario, mathematical modelling has proposed that it may take up to 73 years of cART to eliminate any residual infected cells20,22. The concomitant administration of LRAs to reactivate HIV-1, referred as 'shock', will still rely on a suboptimal natural immune response, responsible for the 'kill', to eliminate the latent reservoir of virus, whereas the cART will protect uninfected cells from becoming infected. Therefore, it is now proposed that HIV-1 envelope-specific monoclonal antibody (mAb)-based strategies, including dual-affinity re-targeting (DART) molecules, can be implemented to provide additional cytotoxic functions (the kill) to the immune responses by mediating the elimination of the latent reservoir during reactivation of the provirus.
In addition to the cellular immune responses elicited by natural infection, antibody responses contribute to the landscape of virus evolution within the infected individuals. Notably, neutralizing antibody responses exert immune pressure following acute HIV-1 infection8,9,10 and select for immune variants of the HIV-1 envelope (HIV-1 Env) that in turn promote the development of broadly autologous neutralizing antibodies11. This co-evolution has also been demonstrated during chronic infection12. These autologous neutralizing responses were associated with control of virus replication in a subset of infected individuals after treatment interruption13. Meanwhile, infusion of the CD20-specific monoclonal antibody (mAb) rituximab resulted in loss of control of virus replication14. This effect occurred because rituximab depleted B cells and consequently led to the loss of autologous neutralizing responses14. Together, these studies provide a rationale for identifying natural antiviral mechanisms that can be enhanced and leveraged to eliminate latently infected CD4+ T cells and thus cure HIV-1 infection15,16,17.
The implementation of cART can arrest the clinical progression of HIV-1 infection, increase life expectancy and improve the quality of life of HIV-1-infected individuals. However, HIV-1 still persists as a transcriptionally quiescent provirus even when cART has been administered for more than 10 years18,19,20,21 (Fig. 1a). A model of the decay of the pool of latently infected CD4+ T cells estimated that it would require at least 73 years of continual cART to ablate this reservoir20,22. Despite the inability of cART to eliminate the latent reservoir, immediate initiation of cART during the acute phase of infection can reduce the size of the reservoir. Indeed, a study using a quantitative viral outgrowth assay (QVOA) demonstrated this effect; the early initiation of cART reduced the frequency of cells harbouring replication-competent virus23.
Thus, these data indicate that immune responses and cART can constrain the pool of latently infected CD4+ T cells, but eradication of the latent T cell pool will require novel immune intervention strategies. In this regard, substantial progress has recently been made. HIV-1-specific mAbs and engineered immunotherapeutics that are based on mAb specificities and cellular immune mechanisms have been developed to target the pool of latently infected CD4+ T cells. Here, we discuss how innovative engineered bispecific and trispecific antibodies can be utilized in the context of experimental HIV-1 treatment and cure strategies. In particular, we focus on the novel mAb-based dual-affinity re-targeting (DART) proteins.
Establishment of latent infection
HIV-1 integration during the infective cycle. The acute phase of HIV-1 infection is characterized by rapid replication of the virus within CD4+ T cell subsets and a profound depletion of CD4+ T cells, predominantly in secondary lymphoid organs in the gastrointestinal tract24. In particular, HIV-1-specific CD4+ T cells are targeted by the virus while the T cells acquire antigen specificity during the transition from naive to effector and memory T cells25,26. Acute infection triggers a high level of activation of the immune system, including the release of pro-inflammatory cytokines27. Thus, the overall T cell compartment is also affected by bystander activation, resulting in infection of both naive and memory cells26. HIV-1 can therefore integrate into the genome of a broad population of activated proliferating CD4+ T cells. Interestingly, CD4+ T follicular helper (TFH) cells are considered the main source of cells that support active virus replication in viraemic and treated aviraemic patients during chronic HIV-1 infection28,29. These findings suggest that TFH cells might, therefore, become part of the pool of latently infected cells in the lymph nodes. Both the development of anti-HIV-1 host immune responses30,31,32 and initiation of cART can provide partial control of virus replication while immune system activation declines33,34,35. Notably, CD8+ T cell responses may be prevented from reaching infected TFH target cells within lymph node B cell follicles36. After cART initiation, some activated cells enter the resting phase of the memory response while harbouring integrated and transcriptionally silent provirus. It is this population of resting cells that has been defined as the latent HIV-1 reservoir18 (Fig. 1b).
The frequency of latently infected CD4+ T cells was initially estimated to be approximately 0.05% (500 per 1 × 106) of resting CD4+ T cells, with similar frequencies in lymph nodes and blood19. In patients on antiretroviral therapy for more than 30 months, it was estimated that 0.2–16.2 per 1 × 106 resting CD4+ T cells could harbour provirus20. This absolute frequency of latent HIV-1 infection as measured by QVOA has now been shown to be only a minimal estimate. Ho and collaborators determined that <1% of integrated proviruses could be induced to release infectious progeny upon maximal in vitro stimulation37. Moreover, only a subset (∼12%) of the non-inducible provirus-containing CD4+ T cells had intact HIV-1 genomes and normal long terminal repeat function37. However, there are discrepancies among the different assays used to measure the size of the reservoir, which is attributed to the presence of CD4+ T cells harbouring non-integrated replication-competent virus38. Additional studies are needed to determine the most sensitive methods to quantify the latent reservoir to accurately determine the success of HIV-1 cure strategies39.
Infected cellular subsets that become the reservoir of latently infected CD4+ T cells. Subsets of CD4+ cells are the natural targets of HIV-1. Resting CD4+ T cells that harbour the provirus primarily express the memory cell subset marker CD45RO19. This finding indicates that memory cells are the principal component of the pool of latently infected cells. Further analyses revealed that both central and transitional memory CD4+ T cells constitute reservoirs of persistent HIV-1 (Ref. 40). Measurements of integrated HIV-1 DNA in CD4+ T cells have demonstrated that the reservoir is represented by 93.8% memory cells and 6.2% naive cells in the lymph nodes and by 70.1% memory cells and 20.1% naive cells in the periphery25. QVOA analysis of replication-competent virus identified resting central memory CD4+ T cells as a stable subset of latently infected cells compared with transitional memory cells, which may not represent a persistent reservoir41 because of their shorter lifespan. The frequency of central memory CD4+ T cells harbouring replication-competent virus ranged from 0.03 to 9.74 IUPM in a cohort of cART-treated acutely and chronically HIV-1-infected individuals41. Interestingly, these results supported previous estimates that the frequency of latently infected memory CD4+ T cells is <1 IUPM19,42.
Other cell populations in the immune system in addition to the resting CD4+ T memory cell subsets may contribute to the pool of latently infected cells. Resting naive CD4+ T cells have been identified as potential contributors to the latent HIV-1 reservoir43,44, although it remains unclear how persistent viral latency is within this T cell reservoir. Macrophages can be infected by HIV-1 and have been identified as carriers of the virus in the CNS of patients45. Macrophages are also the main source of HIV-1 production in rhesus macaques infected with simian/HIV (SHIV) chimeric virus during a period of profound CD4+ T cell depletion46. The persistence of true viral latency within the macrophage population has not yet been delineated. It is possible that tissue-resident monocyte-derived cells can represent a component of the pool of latently infected cells. Meanwhile, pluripotent haematopoietic cells are susceptible to HIV-1 infection via CXC-chemokine receptor 4 (CXCR4)47, and in vitro experiments have shown that these cells can become latently infected48,49. However, the contribution of pluripotent CD34+ cells to the pool of latently infected cells has been challenged by Durand and collaborators, who could not detect integrated HIV-1 DNA in highly purified cell preparations50. Therefore, the persistence of viral latency in cellular subsets other than CD4+ T cells and their contribution to the total latent reservoir in vivo currently remains unknown51.
Maintenance of latently infected CD4+ T cells. One explanation for the establishment of the pool of latently infected cells is that a portion of CD4+ T cells survives the cytopathic effect of virus replication following acute infection. This portion of CD4+ T cells returns to a resting stage, resulting in latency. To maintain proviral latency, several molecular mechanisms restrict proviral expression. Epigenetic silencing mechanisms, including restrictions associated with deacetylated histones and methylated histones, are key regulators of proviral repression. In addition, the following non-epigenetic factors influence viral quiescence: the regulation of HIV-1 transcription and elongation by Tat; transcriptional interference; the sequestration of transcription initiation factors; the regulation of positive transcription elongation factor b (p-TEFb); and the restriction of HIV RNA transport52,53,54.
The proviral integration site can also affect the ability of HIV-1 to start a new replication phase upon subsequent activation of the cellular subsets of interest55. Although HIV-1 does not specifically target a unique integration site within the human genome, proviral integration sites are disproportionally found in genes related to T cell homeostasis, such as BTB domain and CNC homologue 2 (BACH2), and cell growth, such as MKL1/myocardin like 2 (MKL2)43,56,57. In addition, HIV-1 integration sites have been identified in proximity to the Alu sequences, suggesting that HIV-1 may not necessarily target genes that are transcriptionally active at the time of infection48.
In summary, there are two potential mechanisms by which the pool of latently infected CD4+ T cells is maintained. The first hypothesis is that the reservoir is maintained through the proliferation of clonally expanded CD4+ T cells58. The second hypothesis is that only 10% of cells in the latent reservoir harbour a single integration site and this population declines with a longer half-life during cART. Therefore, the cells with a single integration site might be those responsible for the majority of the rebounding of the latent reservoir48. Thus, determination of HIV-1 integration sites may lead to new strategies for the reversal and clearance of HIV latency during cART.
Immune responses to eliminate latency
The limited ability of the immune system to recognize HIV-1-infected cells following reactivation from latency represents a hurdle in the elimination of the latent HIV-1 reservoir59. The following factors are thought to contribute to this hurdle: the low level of HIV-1-specific circulating CD8+ T cells in patients on cART59; the inability of reactivated cells to deliver sufficient activation stimuli for the human leukocyte antigen (HLA) class I-restricted antigen-specific effector cells53,59,60; the inadequate level of expression of HIV-1 antigens by the reactivated cells51; and the localization of reactivated cells in protected sanctuary sites such as lymph node B cell follicles29,36. Moreover, proviral sequences in individuals who started cART during the chronic phase of infection carry cytotoxic T lymphocyte escape mutations that may reduce CD8+ T cell-mediated elimination of latently infected cells61.
Viral diversity and clearance strategies
The immune responses elicited during the acute phase of infection are responsible for exerting immune pressure on HIV-1 and inducing the evolution of virus sequences within each infected individual. This effect has been demonstrated to be an outcome of both cellular31,62,63,64,65,66 and humoral responses8,9,10,12,67,68, and the immune system struggles to adapt to the virus evolution to confer protection from disease progression. Analyses of sequences of integrated virus in circulating activated and resting CD4+ T cells from HIV-1-infected patients on cART indicated that the provirus was represented by a single intermixing genetic population69. In another study, when the sequence of the provirus from circulating resting CD4+ T cells was compared with that of plasma virus, the results indicated that plasma virus was genetically distinct from integrated provirus69. Although this finding has not been universally seen in all patients70 and may be due to undersampling, it has raised the possibility of a source of residual virus that is distinct from the resting CD4+ T cell population. Under this premise, the early initiation of cART could limit the diversity of proviral sequences, limit escape mutations and improve the ability of the immune system to clear infected cells. A recent analysis of virus sequences obtained at 0, 3 and 6 months post-initiation of cART in three patients demonstrated that virus replication persists when the plasma virus load is <48 copies per ml71. This persistent virus replication may contribute to changes in the sequence diversity of the provirus in the cellular reservoir71. However, these findings are in contrast to previous observations that indicated a lack of virus evolution in the periphery72,73 and in gut-associated lymphoid tissues73 in individuals on cART74. Nevertheless, the possibility of virus replication persisting below detectable levels and fostering persistent genetic instability of the reservoir (Fig. 1b) represents substantial challenges. That is, it would be particularly challenging for the immune system to adjust to sequence evolution even if it seems that the patient is successfully treated with cART. These data highlight the need for antiretroviral drug regimens that are able to reach sanctuary sites of virus replication and at effective concentrations to inhibit virus replication. More studies are needed to confirm these findings.
The reversal of latency in a safe and fully effective manner across the biological and anatomical diversity of the HIV reservoir is a daunting task53,60. Initial proposals to reverse latency included stimulation of the immune system in patients on suppressive cART through the administration of a combination of the CD3-specific mAb OKT3 with anti-CD28 (Ref. 21) or with interleukin-2 (IL-2)75. Data from these initial studies indicated that the co-administration of OKT3 and IL-2 was toxic and elicited anti-OKT3 antibody responses. Moreover, despite strong activation of the immune system, the combination failed to reduce the number of latently infected CD4+ T cells.
More recent studies of latency reversing agents (LRAs) have included those that inhibit the silencing effects of epigenetic and non-epigenetic restrictions54. These types of LRAs induce proviral expression but without overt cellular activation54. Several compounds have been identified that can directly or indirectly reverse epigenetic silencing. Compounds that can directly reverse epigenetic silencing include inhibitors of histone deacetylase (HDAC), histone methyltransferase (HMT) and DNA methylase (DNMT), and have been studied in vitro, ex vivo or in clinical trials76. Clinical studies of HDAC inhibitors have suggested that even a single dose of the HDAC inhibitor vorinostat could disrupt latency77 (Fig. 1a,b). This study opened the door to more targeted approaches to reverse latency in the absence of high-level immune activation in the hope of achieving clearance of latently infected cellular reservoirs. Although several studies have demonstrated the ability of HDAC inhibitors to reverse latency, at least transiently78,79,80,81,82, none has demonstrated that induction of proviral expression results in the depletion of the pool of latently infected cells. Optimal dosing of LRA regimens may need further refinement because a dampened therapeutic response has been observed in the patients treated with daily doses of vorinostat83. In an encouraging study by Archin and collaborators84, a HMT inhibitor led to a reduction in the trimethylation of histone 3 at lysine 27 (H3K27) of the HIV provirus; this result has spurred the development of LRAs that could act on other epigenetic restrictions. The possibility of combining more than one LRA is now being explored78,80.
Overcoming limitations of immune responses
Novel strategies are needed to achieve HIV-1 cure because neither host immune responses59,61 nor a combination of LRA plus cART60,71,85 are effective in reducing the size of the pool of latently infected cells. In addition to the mechanism of virus escape31,63, the limited efficiency of CD8+ T cell responses is partially attributed to the exhaustion of these cells. This finding has been demonstrated by the expression of cellular exhaustion markers such as programmed cell death protein 1 (PD1)86,87,88. Initiation of cART after infection is not capable of fully reverting the exhausted functional phenotype of CD8+ T cells89, which could represent a limitation of HLA class I-restricted antigen-specific cells to kill latently infected cells. mAbs and mAb-based therapeutics capable of mediating antibody-dependent cellular cytotoxicity (ADCC) by recruiting crystallizable fragment (Fc)-gamma receptor (FcγR) IIIa-bearing cells, such as natural killer cells, are of particular interest17. In addition, the utilization of mAb-based proteins that can recognize infected cells and redirect cytotoxic effector T cells independently of HLA class I restriction and exhaustion level to kill infected cells has become a strategy for developing new immunotherapies.
HIV-1 Env mAb specificities
A wide repertoire of HIV-1 Env-specific mAbs can mediate ADCC independently of their neutralizing functions90. This ability is due to the recognition by mAbs of conformational states of the HIV-1 Env glycoproteins expressed on virus-infected cell membranes that may be different from those present on virions91,92 (Fig. 2a). The conformational state of the functional HIV-1 Env glycoprotein on infectious virions is a 'closed' trimeric structure of the assembled gp120–gp41 complex. These functional trimers contain numerous epitopes that can be targeted by broadly neutralizing antibodies (bNAbs) (Fig. 2b). These epitopes include the CD4-binding site (CD4bs), variable regions 1 and 2 (V1V2), glycans at the base of variable region 3 (V3), the gp120-gp41 interface, and the membrane external proximal region (MPER) (Fig. 2b). VRC01 (Ref. 93), VRC07 (Ref. 94), b12 (Ref. 95) and 3BNC117 (Ref. 96) are bNAbs that target CD4bs, whereas PG9 and PG16 (Ref. 97) target V1V2. The bNAbs 10–1074 (Ref. 98) and PGT121 (Ref. 99) target glycans at the base of V3, whereas PGT151 targets the gp120-gp41 interface100. Finally, the bNAb 10e8 targets the MPER101 (Fig. 2b).
a | HIV-1 envelope proteins can be expressed in different conformational structures referred to as closed or open trimers, monomers and stumps. b | Each envelope conformation is susceptible to recognition by monoclonal antibodies (mAbs) defined as those with neutralizing (nAbs) and non-neutralizing (non-nAbs) activity. All mAbs depicted in the figure have already been used to treat simian/HIV (SHIV) infection in animal models or HIV-1 infection in pilot clinical trials. c | These mAbs have been used to engineer new molecules such as bispecific mAbs (top) or dual-affinity re-targeting (DART) proteins (bottom) with the goal of improving the recognition of HIV-1 Env on the surface of infected cells and to recruit effector cells to mediate their clearance. CD4bs, CD4 binding site; MPER, membrane external proximal region; V, variable region; VH, variable heavy chain; VL, variable light chain.
Additional epitopes on the virion can be exposed upon engagement of the cellular CD4 receptor102 or as a result of the HIV-1 Env adopting different conformational states, commonly referred to as 'breathing motions' of the viral envelope proteins103,104,105. This change in conformation leads to a more open envelope arrangement that exposes CD4-inducible epitopes102 in the C1/C2 region of gp120 as well as the HR1-HR2 region of gp41. These regions can be recognized by the HIV-1 Env non-neutralizing mAbs A32 (Refs 106,107) and 7B2 (Refs 108,109,110). The A32 mAb targets the conformational CD4-inducible C1/C2 epitope, the earliest epitope to be exposed during cell–cell infection111,112,113, whereas 7B2 targets the HR1-HR2 region of gp41. A key feature of immunotherapeutic mAb specificities is their high-affinity recognition of conserved HIV-1 Env structures on the surface of infected cells. Maximizing the sensitivity for binding to low levels of cell surface envelope glycoproteins upon exposure to an LRA, as well as the kinetics of epitope exposure and recognition, is critical for the design of curative strategies based on HIV-1 Env-specific mAbs. Utilization of mAbs with specificities that can also neutralize virions, in addition to targeting HIV-1 Env on infected cells, will be of additional benefit for clearing virions or limiting infectious virion spread.
Antibodies for treating HIV-1 infection
In the one partially efficacious HIV-1 vaccine trial to date, high levels of antibodies that mediated ADCC, but were not broadly neutralizing, correlated with decreased risk of HIV-1 infection114. ADCC has also been associated with delayed onset of overt disease115,116 and with control of virus replication117. Emerging data support the notion of harnessing ADCC-mediating antibodies to attain a HIV cure because such antibodies can be engineered to attack and clear latently infected cells upon reactivation of provirus. Two non-neutralizing ADCC-mediating mAbs that show promise for targeting the latent reservoir are 7B2 (Ref. 118) and A32 (Refs 102,107). 7B2 and A32 recognize conserved residues of HIV-1 Env in the gp41 immunodominant and C1/C2 gp120 regions, respectively107,119. Although passive infusion of these two mAbs did not prevent infection, they did limit the number of transmitted/founder (T/F) HIV-1 variants that established infection110. Thus, these types of antibodies are useful as models to design more potent antibody therapeutics that can redirect cytotoxic cells to recognize reactivated latently infected cells.
bNabs administered individually or in combination have been shown to prevent SHIV infection in non-human primates120,121,122,123,124,125,126,127,128. In some studies, the protective effect of the bNAbs also correlated with their Fc-mediated effector functions, including the ability to recruit FcγR-bearing effector cells129,130,131,132,133,134. The importance of mAb-mediated recruitment of FcγR-bearing cells was recently highlighted by Lu and collaborators135. Accelerated clearance of infected CD4+ T cells in a mouse model was observed when the mAb (a variant of 3BNC117, a CD4bs-specific bNAb96) had a functional Fc domain, and was therefore capable of mediating ADCC, but not when the mAb was engineered with an inactivated Fc domain135.
The diversity of functions exhibited by bNAbs that target both virions and infected cells make them candidates for both treatment and cure of HIV-1 infection. Moreover, bNAbs have been demonstrated to suppress viraemia in rhesus macaques chronically infected with SHIV136,137,138. In this model, a cocktail of bNAbs137, including the N332 glycan-dependent PGT121 (Ref. 99), the CD4bs-specific 3BNC117 (Ref. 96) and b12 (Ref. 95) mAbs, reduced the level of plasma viraemia by 3.1 log within 7 days post-infusion. Moreover, the mAb combination reduced proviral DNA levels in secondary lymphoid sites in the gut, lymph nodes and peripheral blood. Control was maintained for up to 56 days after levels of the mAbs dropped below the limit of detection. A second infusion of the PGT121–3BNC117 combination controlled rebounding virus, although the control was less sustained. Evaluation of the neutralization sensitivity of the SHIV-SF162P3 challenge stock suggested that the PGT121 mAb was the most effective in reducing proviral DNA in lymph nodes, gastrointestinal mucosa and peripheral blood in addition to decreasing viral load. Importantly, infusion of the VCR07-PGT121 mAb combination during the acute phase of infection in the SHIV-infected non-human primate model has provided evidence of the effectiveness of these mAbs. This combination was able to reduce peak viraemia and the size of the pool of latently infected cells139 as well as completely control virus replication and prevent the establishment of latently infected cells140. These data raise the possibility that bNAbs could have a considerable impact on the pool of latently infected cells by reducing the level of provirus DNA in tissue compartments. However, further studies are needed to determine whether these reductions in provirus DNA are sustained.
In a recent study16, a combination of bNAbs comprising 3BNC117, 10–1074 (Ref. 98) and PG16 (Ref. 97) was evaluated in humanized mice infected with the HIV-1YU2 isolate. Infusion of this bNAb cocktail after infection resulted in decreased viraemia in ∼50% of the mice, and mice treated with mAbs demonstrated substantially delayed virus rebound compared with mice treated with cART. Moreover, a decline in the level of cellular-associated DNA was observed only in the aviraemic mice treated with the triple mAb cocktail. In the same study, a combination of LRAs (vorinostat, an anti-cytotoxic T-lymphocyte antigen (CTLA) mAb and I-BET151) induced viraemia in the bNAb-treated mice that had previously exhibited undetectable levels of plasma virus load after treatment. Interestingly, cell-associated viral DNA was not detected in the mice that showed no rebound of viraemia, whereas cell-associated viral DNA was detected at an average of 0.09 copies per T cell in the animals that had rebounding viraemia. The mechanism by which this combination of bNAbs was effective in controlling virus replication was probably through the interaction of their Fc domains with FcγRs on effector cells. Animals that received mAbs that had critical mutations in their Fc regions — which abrogated murine and human Fc receptor binding but retained virus neutralization activity — were more likely to have virus rebound at day 44 after the last Ab injection (60% versus 5% for wild-type antibodies). The results of this study support those of the study of SHIV-infected non-human primates137 demonstrating that bNAbs can significantly affect both plasma viraemia and the pool of latently infected cells. That is, bNAbs exert their effects by recognizing HIV-1 Env on the host cell membrane16 and engaging FcγR-bearing cells, as previously shown in humanized mice141. Moreover, these results highlight the importance of engaging FcγR-bearing cells for optimal virus control.
A clinical trial of the 3BNC117 mAb has recapitulated the finding of controlled plasma viraemia142. The longitudinal observation of the patients enrolled in this study revealed a surprising effect on host humoral immune responses; bNAb responses developed that were not present before the infusion of the 3BNC117 mAb143. In addition to these studies, the VRC01 mAb was also tested for its ability to control virus replication in chronically infected individuals144. The administration of a single dose of this mAb induced a reduction of up to 1.8 log10 in plasma viraemia. However, VRC01-resistant virus was observed in two of the eight treated patients before treatment and hampered the therapeutic effect of the mAb. Similarly, the appearance of increased resistance, which indicates escape, was observed in 70% of the 13 individuals treated with 3BNC117 during analytical treatment interruption145. Therefore, it is possible that bNAb-based immunotherapy may positively alter the natural humoral responses and exploit new unexpected outcomes that have to be further evaluated for their impact on both control of virus replication and reduction of the latent reservoir. For the latter effect, further studies are also needed to understand the impact that Fc-mediated functions of bNAbs have on engaging effector cells to clear the latent reservoir.
Engineered antibodies with multiple specificities
Innovations in antibody therapeutics have led to the generation of antigen-binding variable fragments that can be combined into bispecific or trispecific molecules146,147. There are generally two major classes of bispecific and trispecific mAbs: those with or without an antibody Fc region. Bispecific antibodies (bsAbs) are molecules engineered with two antigen-binding variable fragments of immunoglobulins to recognize two separate antigens. These molecules can interact with two different antigens whether they are presented on the surface of an individual cell or on two distinct cells. In the latter case, the molecules will facilitate cellular interactions and provide specific signals for the desired outcome. Several different bsAbs have been designed to diagnose and treat several human diseases, including infectious diseases and cancer148.
BsAbs and HIV-1 infection. Initially, bsAbs were designed to target free HIV-1 virions or HIV-1-infected cells based on gp41-specific antibodies. Studies have demonstrated their ability to mediate virus neutralization149, combined neutralization and ADCC150, and redirection of CD3+ T cells against HIV-1-infected or latently infected cells lines151. A more recent bispecific approach was taken by Sun and collaborators, who designed iMabm36, a bsAb that combines iMab (the CD4-specific mAb ibalizumab) with two copies of m36 (a single domain, neutralizing, HIV gp120-specific antibody)152. iMabm36 exhibited a higher neutralizing capacity than m36 alone; iMabm36 was capable of neutralizing 83% of 118 pseudoviruses with a half-maximal inhibitory concentration (IC50) value of <0.1 μg per ml. A clinical trial has been designed to evaluate the activity of this bsAb for treating HIV-1 infection.
The most recent analyses of the potency and breadth of bNAbs against HIV-1 prompted studies aimed at designing an immunoglobulin with an antigen-binding fragment that can independently recognize two different HIV-1 Env epitopes while having a common Fc region (Figs 2c,3a). Four independent bsAbs were developed using combinations of bNAbs described in the previous section: VRC07 × 10e8, VRC07 × PGT121, VRC07 × PG9-16 and 10e8 × PG9-16 (Ref. 153). The in vitro neutralization profiles of these bsAbs at 25 μg per ml revealed that they retained the breadth of both parental bNAbs, neutralizing >94% of the pseudoviruses representing 206 HIV-1 isolates. The most promising bispecific combination was VRC07 × PG9-16, which neutralized >84% of pseudoviruses with an IC50 of <1 μg per ml. Moreover, this bsAb combination neutralized all ten pseudoviruses resistant to VRC07 or PG9-16 alone. Notably, VRC07 × PG9-16 displayed 3-fold to 37-fold increased potency compared with the individual mAbs against 8 out of 10 resistant pseudoviruses included in the panel tested. Studies using uninfected non-human primates did not reveal any difference in the pharmacokinetics between the parental bNAbs and the bsAbs. This result represents an encouraging advance in designing bsAbs that could be used for the treatment and/or cure of HIV-1 infection.
a | Monoclonal and bispecific antibodies engineered with the crystallizable fragment (Fc) region will engage Fcγ receptor (FcγR)-bearing cells such as natural killer cells, monocytes and macrophages, polymorphonucleated cells and γ/δ T cells. b | Dual-affinity re-targeting (DART) proteins can be engineered with an effector arm based on CD3-specific or CD16-specific antibodies that are capable of binding CD3+ T cells or FcγR-bearing cells, respectively. Env, envelope; VH, variable heavy chain; VL, variable light chain.
Bispecific BiTE and DART molecules. bsAbs have been engineered so that one arm recognizes antigens on the surface of target cells while the second arm recognizes functional receptors on the surface of effector cells to mediate target-specific redirected killing activity. The first generation of molecules, termed bispecific T cell engagers (BiTEs), consisted of two single-chain variable fragments from different antibodies joined by a single polypeptide linker154. One fragment was designed to bind CD3+ T cells (CD4+ or CD8+) via the CD3 receptor and the other fragment was designed to bind a tumour cell via a tumour-specific antigen. The redirected T cell killing of antigen-expressing target cells by bsAbs is concomitant with effector T cell activation, proliferation and upregulation of granzyme B and perforin155,156. Moreover, the effects of bsAbs are induced in a manner that is independent of major histocompatibility complex I (MHC I) or co-stimulatory molecules155,156. These bispecific T cell-redirecting molecules are reported to be effective in vivo at doses several-fold lower than those typically required for mAbs157. Multiple BiTE molecules have advanced into clinical development for the treatment of malignancies148, and blinatumomab (a CD19 × CD3 BiTE) was approved in 2014 by the US Food and Drug Administration (FDA) for the treatment of acute lymphoblastic leukaemia158.
The dual-affinity re-targeting (DART) scaffold is a novel bsAb-based modality that offers improved stability, manufacturability and potency compared to the BiTE format159,160,161. In DART molecules, the variable domains of the two antigen-binding moieties are incorporated into a disulfide-linked heterodimer. Short linkers between the variable light chain and variable heavy chain segments of this heterodimer promote a 'diabody'-type association, with the disulfide bond stabilizing the structure (Figs 2c,3b).
Moore and collaborators generated and compared side-by-side the in vitro performance of CD19 × CD3 BiTE and DART proteins based on the same anti-human CD3 and anti-human CD19 sequences as blinatumomab160. The crystal structure of a disulfide-constrained DART molecule showed that it assembles into a novel compact structure, with the two antigen-binding sites separated from each other by approximately 30 Å and facing approximately 90° apart162. The orientation and short distance between the antigen-binding domains of the DART protein may facilitate more efficient synapse formation between the T cell and the target cell and contribute to an increase in potency of T cell-directed lysis compared with other bsAb formats. Therefore, it was suggested that the more rigid and compact conformation of the DART protein compared to the BiTE protein could be responsible for the higher potency of DART proteins in redirecting T cell killing activity and inducing T cell activation159-161. These data make the case for the evaluation of DART molecules that redirect T cells to target and destroy HIV-1-infected cells.
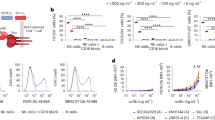
DART molecules targeting HIV-1 Env-expressing cells. As the combination of an LRA with cART is not sufficient to reduce the size of the pool of latently infected cells, additional strategies are needed to mediate the clearance of the HIV-1 Env-expressing infected cells. Sung and colleagues designed DART molecules with HIV-1 Env and CD3 specificities and evaluated these molecules for their capacity to recruit and redirect cytotoxic T effector cells to infected CD4+ cells expressing HIV-1 Env on their surface119. The HIV-1-specific arm was based on the A32 and 7B2 non-neutralizing mAbs, which target the CD4-inducible C1/C2 and gp41 cluster I antigens, respectively119. The T cell-binding arm was based on a mAb that targets the CD3ɛ component of the T cell receptor complex. In vitro studies of lymphocytes from HIV-1 seronegative individuals demonstrated that the A32 × CD3 and 7B2 × CD3 DART proteins were capable of recruiting up to 25% of the total resting CD3+CD8+ population at a 50% effective concentration (EC50) of <1 and 10 ng per ml, respectively. When lymphocytes from HIV-1 seropositive patients were stimulated with an LRA (vorinostat) or a mitogen (phytohaemagglutinin) and then incubated with the DART molecules, the virus recovered in supernatants was substantially reduced (by 40–100%). A combination of the two DART molecules was always as effective as the most potent individual DART molecule in each patient sample. This finding suggests that DART molecules do not seem to exhibit antagonistic effects and that combinations of DART molecules may be an effective way to increase the breadth of activity against diverse HIV-1 isolates in the clinical setting. Interestingly, when degranulation of T cells from HIV-1-infected individuals was characterized in vitro, it was noted that both CD8+ T cells and uninfected CD4+ T cell were engaged by the CD3 specificity of the DART molecule and degranulated. These results suggest that CD4+ T cells contribute to the cytotoxic effects mediated by the DART molecule. Moreover, CD4+ T cells can act as effector cells and support the elimination of latently infected cells even in absence of the effector CD8+T cell subsets, although the latter subsets are more proficient in promoting cytolysis.
A subsequent study by Sloan et al.163 evaluated HIV × CD3 DART molecules in which the HIV-specific arm was based on bNAbs that target the N332-glycan (PGT121)99, V1V2 (PGT145)164, the CD4 binding site (CD4bs) (VRC01)93 and MPER (10e8)101. Of these bNAb-based DART molecules, PG121 × CD3 exhibited the highest potency when tested against CD4+ T cells infected with diverse HIV-1 isolates; its potency was comparable to that of A32 × CD3 and 7B2 × CD3 (EC50 values: 4.2–5.5 pM for A32 × CD3, 1.9–20.5 pM for 7B2 × CD3, and 0.2-24pM for PGT121 × CD3). Paired combinations of these three DART molecules revealed that neither antagonistic nor apparent synergistic effects were observed for their cytotoxic activity, which was probably due to the high level of potency of the individual molecules under the given assay conditions. Notably, when cells from HIV-1-infected patients were incubated with the protein kinase C agonist indolactam to reactivate virus replication, the DART molecules mediated >74% virus reduction in two out of four patient samples. It is theoretically possible that the CD3-directed portion of the DART molecule may stimulate infected CD4+T cells and thereby facilitate viral spread. However, data from Sung et al.119 and Sloan et al.163 indicated that no increase in virus replication occurred when DART molecules were incubated with infected CD4+ cells in the absence of CD8+ effector cells. Together, these two studies119,163 demonstrate the in vitro potency of HIV × CD3 DART proteins and provide a strong foundation for the testing of these first-generation DART proteins in clinical trials.
A different HIV × CD3 bispecific molecule, in which the anti-HIV arm was based on a bNAb targeting the CD4bs (VRC07)94, was evaluated by Pegu and collaborators165. The structure of this bispecific molecule differed from that of DART molecules in that a CD3-specific single-chain variable fragment arm was linked to the light chain of the antigen-binding fragment of the VRC07 mAb. This protein was named VRC07-αCD3 (Ref. 165). Similar to the DART molecules, VRC07-αCD3 did not induce activation of either CD8+ or CD4+ T cells in the absence of target cells expressing HIV-1 Env. The investigators determined that VRC07-αCD3 directed the cytotoxic activity of resting CD8+ T cells obtained from healthy HIV-1 seronegative donors against several cell lines infected with HIV-1. This experiment represented models for constitutive or inducible HIV-1 expression, with the latter considered as a model for in vitro latency. Notably, VRC07-αCD3 was also tested for its ability to recognize latently infected cells present in peripheral blood mononuclear cells obtained from eight HIV-1-infected donors who were on suppressive cART. After 2 days of incubation with VRC07-αCD3 in the absence of any other stimulation or effector cells, the CD4+ T cells from all donors expressed HIV-1 Env on their surface. Most importantly, the frequency of proviral DNA-positive CD4+ T cells was decreased in the cultures of five out of eight subjects165.
Future directions
The path forward for identifying superior molecules for HIV-1 cure will require an in-depth understanding of how to effectively target infected cells that express low levels of HIV-1 Env. In addition, the molecules must be able to access difficult-to-reach anatomical sites harbouring the latent virus reservoir. Subsequent engineering improvements of these molecules are also needed to enhance the capacity to engage the most potent effector cells and to achieve a longer in vivo half-life. Preclinical studies conducted in the SHIV-infected non-human primate model have indicated that administration of recombinant adeno-associated virus vectors engineered to express a CD4-immunoglobulin fusion protein in combination with a small CCR5-mimetic sulfopeptide appear to be well tolerated166. Moreover, this construct was capable of protecting the animals from multiple challenges of SHIV-AD8 for up to 40 weeks166. These results provide encouraging initial observations on the safety of long-term administration of a mAb-derived molecule. Nevertheless, the safety, immunogenicity potential and, ultimately, efficacy of these novel proteins will have to be addressed by appropriate clinical studies.
It is clear that peripheral blood and secondary lymphoid organs are not the only sites that harbour latently infected cells. Bone marrow or the CNS may also represent a sanctuary component of the HIV-1 reservoir167,168 and would need to be effectively targeted by immunotherapeutics. Encouraging results from in vitro analyses of DART molecules with different HIV-1 Env specificities suggest that they may have additive effects119,163 when combined, which may help eliminate the impact of virus mutations. Improved effector function by DART molecules may be achieved with an effector cell-binding arm based on a CD16-specific (FcγR IIIa) antibody instead of the CD3-specific antibody (Fig. 3b). A combination of HIV DART molecules with different effector arms would facilitate the engagement of different effector cell populations (for example, natural killer cells and cytotoxic T cells) that may differentially migrate to the anatomical sites where infected target cells reside. Moreover, there have been promising improvements in the in vivo half-life160-163. Incorporation of an engineered Fc domain with L234A/L235A amino acid substitutions that prevent binding to activating FcγRs but retain neonatal Fc receptor binding increased the half-life of the DART molecule while maintaining potent killing of HIV-1-infected CD4+ T cells163.
Ongoing studies are also exploring molecules beyond bispecificity to determine whether the generation of trispecific or tetraspecific antibody-derived molecules against HIV-1 could achieve better antiviral activity, in a similar manner as has been reported for an anticancer trispecific antibody146,147.
Along with efforts to improve the current proteins and to design novel proteins capable of recognizing infected cells, the field is also exploring proteins that can rescue exhausted effector cells. Examples include agents that can interfere with the interaction of PD1 and/or CTLA4 with their ligands169,170,171, and novel antibody-based proteins that incorporate such agents.
Finally, even with improvements in these therapeutic agents and enhancements in immune targeting, it will be important to find the necessary strategies that can disrupt the sanctuaries of virus replication36 that remain inaccessible.
Conclusions
Bispecific and trispecific antibodies with multiple broadly reactive anti-HIV-1 specificities have been developed to augment the breadth of recognition and neutralization of diverse HIV-1 isolates. In addition, novel bsAb-derived molecules, such as those in BiTE or DART formats, have been designed to co-engage HIV-1 Env-expressing target cells with cytotoxic effector cells. The aim of BiTE and DART molecules is to enhance the clearance of latently infected CD4+ T cells upon HIV-1 provirus reactivation through natural or drug-induced means. These novel immunotherapeutics are based on high-affinity HIV-1 Env-specific mAbs that may be either neutralizing or non-neutralizing. The key feature is that they must be directed against broadly conserved regions of cell surface-expressed virus antigen to expand the breadth of recognition of cells infected with diverse HIV-1 isolates10. A major advantage of these immunotherapeutic molecules that redirect effector cells to antigen-expressing targets is that they are highly potent. Moreover, they will engage cytotoxic cellular subsets independently of antigen HLA class restriction and T cell receptor clonal specificity. In addition to the initial in vitro observations, preclinical studies using appropriate animal models will be needed to document safety and to support their clinical development for the treatment of HIV-1 infection. Current and planned clinical trials utilizing these exciting new reagents will provide important new insights into the development of innovative immunotherapeutic regimens for HIV-1 cure.
References
Allers, K. et al. Evidence for the cure of HIV infection by CCR5 32/ 32 stem cell transplantation. Blood 117, 2791–2799 (2011).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369, 1828–1835 (2013).
Fauci, A. S., Marston, H. D. & Folkers, G. K. An HIV cure: feasibility, discovery, and implementation. JAMA 312, 335–336 (2014).
Walker, B. & McMichael, A. The T-cell response to HIV. Cold Spring Harb. Perspect. Med. 2, a007054 (2012).
Hatano, H. et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J. Virol. 83, 329–335 (2009).
Pereyra, F. et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200, 984–990 (2009).
Blankson, J. N. et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 81, 2508–2518 (2007).
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003).
Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl Acad. Sci. USA 100, 4144–4149 (2003).
Liao, H.-X. et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496, 469–476 (2013).
Liao, H.-X. et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353, 268–282 (2006).
Moody, M. A. et al. Strain-specific V3 and CD4 binding site autologous HIV-1 neutralizing antibodies select neutralization-resistant viruses. Cell Host Microbe 18, 354–362 (2015).
Montefiori, D. C., Hill, T. S., Vo, H. T. T., Walker, B. D. & Rosenberg, E. S. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J. Virol. 75, 10200–10207 (2001).
Huang, K.-H. G. et al. B-Cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat. Commun. 1, 1–7 (2010).
Jones, R. B. & Walker, B. D. HIV-specific CD8+ T cells and HIV eradication. J. Clin. Invest. 126, 455–463 (2016).
Halper-Stromberg, A. et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158, 989–999 (2014).
Lee, W. S., Parsons, M. S., Kent, S. J. & Lichtfuss, M. Can HIV-1-specific ADCC assist the clearance of reactivated latently infected cells? Front. Immunol. 6, 265 (2015).
Chun, T.-W. et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1, 1284–1290 (1995).
Chun, T.-W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997).
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Wong, J. K. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997).
Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9, 727–728 (2003).
Archin, N. M. et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc. Natl Acad. Sci. USA 109, 9523–9528 (2012).
Brenchley, J. M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Josefsson, L. et al. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathog. 9, e1003432 (2013).
Douek, D. C. et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417, 95–98 (2002).
Stacey, A. R. et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83, 3719–3733 (2009).
Banga, R. et al. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 22, 754–761 (2016).
Perreau, M. et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 210, 143–156 (2013).
Safrit, J. T., Andrews, C. A., Zhu, T., Ho, D. D. & Koup, R. A. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J. Exp. Med. 179, 463–472 (1994).
Goonetilleke, N. et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206, 1253–1272 (2009).
Tomaras, G. D. & Haynes, B. F. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr. Opin. HIV AIDS 4, 373–379 (2009).
Gay, C. et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS ONE 6, e19617 (2011).
Gay, C. L. et al. Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection. AIDS 25, 941–949 (2011).
Vinikoor, M. J. et al. Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activation. J. Acquir. Immune Defic. Syndr. 62, 505–508 (2013).
Fukazawa, Y. et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 21, 132–139 (2015).
Ho, Y.-C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013).
Spina, C. A. et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 9, e1003834 (2013).
Massanella, M. & Richman, D. D. Measuring the latent reservoir in vivo. J. Clin. Invest. 126, 464–472 (2016).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900 (2009).
Soriano-Sarabia, N. et al. Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J. Virol. 88, 14070–14077 (2014).
Eriksson, S. et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9, e1003174 (2013).
Maldarelli, F. et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014).
Bruner, K. M., Hosmane, N. N. & Siliciano, R. F. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 23, 192–203 (2015).
Gartner, S., Markovits, P., Markovitz, D. M., Betts, R. F. & Popovic, M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA 256, 2365–2371 (1986).
Igarashi, T., Brown, C. R. & Endo, Y. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl Acad. Sci. USA 98, 658–663 (2001).
Carter, C. C. et al. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe 9, 223–234 (2011).
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160, 420–432 (2015).
Carter, C. C. et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 16, 446–451 (2010).
Durand, C. M. et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J. Infect. Dis. 205, 1014–1018 (2012).
Eisele, E. & Siliciano, R. F. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37, 377–388 (2012).
Siliciano, R. F. & Greene, W. C. HIV latency. Cold Spring Harb. Perspect. Med. 1, a007096 (2011).
Ruelas, D. S. & Greene, W. C. An integrated overview of HIV-1 latency. Cell 155, 519–529 (2013).
Mbonye, U. & Karn, J. Control of HIV latency by epigenetic and non-epigenetic mechanisms. Curr. HIV Res. 9, 554–567 (2011).
Sherrill-Mix, S., Lewinski, M. K. & Famiglietti, M. HIV latency and integration site placement in five cell-based models. Retrovirology 10, 1–14 (2013).
Blankson, J. N., Persaud, D. & Siliciano, R. F. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53, 557–593 (2002).
Deeks, S. G. et al. Towards an HIV cure: a global scientific strategy. Nat. Rev. Immunol. 12, 607–614 (2012).
Simonetti, F. R. et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl Acad. Sci. USA 113, 1883–1888 (2016).
Shan, L. et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501 (2012).
Archin, N. M., Sung, J. M., Garrido, C., Soriano-Sarabia, N. & Margolis, D. M. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat. Rev. Microbiol. 12, 750–764 (2014).
Deng, K. et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385 (2015).
Liu, M. K. P. et al. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. J. Clin. Invest. 123, 380 (2013).
Ferrari, G. et al. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog. 7, e1001273 (2011).
Streeck, H. et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5, e100 (2008).
Streeck, H. et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J. Virol. 83, 7641–7648 (2009).
Turnbull, E. L. et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J. Immunol. 182, 7131–7145 (2009).
Mahalanabis, M. et al. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J. Virol. 83, 662–672 (2009).
Gao, F. et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158, 481–491 (2014).
Brennan, T. P. et al. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J. Virol. 83, 8470–8481 (2009).
Anderson, J. A. et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J. Virol. 85, 5220–5223 (2011).
Lorenzo-Redondo, R. et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530, 51–56 (2016).
Kearney, M. F. et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 10, e1004010 (2014).
Josefsson, L. et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc. Natl Acad. Sci. USA 110, E4987–E4996 (2013).
Lewin, S. R. et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med. 22, 839–850 (2016).
Prins, J. M. et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13, 2405–2410 (1999).
Kumar, A., Darcis, G., Van Lint, C. & Herbein, G. Epigenetic control of HIV-1 post integration latency: implications for therapy. Clin. Epigenet. 7, 103 (2015).
Archin, N. M. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485 (2012).
Spivak, A. M. et al. Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4+ T cells from aviremic patients. Antimicrob. Agents Chemother. 59, 5984–5991 (2015).
Rasmussen, T. A. et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1, e13–e21 (2014).
Bouchat, S. et al. Sequential treatment with 5-aza-2′-deoxycytidine and deacetylase inhibitors reactivates HIV-1. EMBO Mol. Med. 8, 117–138 (2015).
Elliott, J. H. et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 10, e1004473 (2014).
Søgaard, O. S. et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 11, e1005142 (2015).
Archin, N. M. et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J. Infect. Dis. 210, 728–735 (2014).
Tripathy, M. K., McManamy, M. E. M., Burch, B. D., Archin, N. M. & Margolis, D. M. H3K27 demethylation at the proviral promoter sensitizes latent HIV to the effects of vorinostat in ex vivo cultures of resting CD4+ T cells. J. Virol. 89, 8392–8405 (2015).
Durand, C. M., Blankson, J. N. & Siliciano, R. F. Developing strategies for HIV-1 eradication. Trends Immunol. 33, 554–562 (2012).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Trautmann, L., Janbazian, L., Chomont, N. & Said, E. Upregulation of PD-1 expression on HIV-specific CD8 T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202 (2006).
Yamamoto, T. et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 117, 4805–4815 (2011).
Jensen, S. S. et al. Initiation of antiretroviral therapy (ART) at different stages of HIV-1 disease is not associated with the proportion of exhausted CD8+ T cells. PLoS ONE 10, e0139573 (2015).
Pollara, J. et al. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr. HIV Res. 11, 378–387 (2013).
Poignard, P. et al. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77, 353–365 (2003).
Moore, P. L. et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80, 2515–2528 (2006).
Zhou, T. et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817 (2010).
Diskin, R. et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334, 1289–1293 (2011).
Burton, D. R. et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266, 1024–1027 (1994).
Scheid, J. F. et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333, 1633–1637 (2011).
Walker, L. M. et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289 (2009).
Mouquet, H. et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl Acad. Sci. USA 109, E3268–E3277 (2012).
Walker, L. M. et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 (2011).
Blattner, C. et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40, 669–680 (2014).
Huang, J. et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515, 138–142 (2014).
Wyatt, R. et al. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69, 5723–5733 (1995).
Lewis, J. K., Bothner, B., Smith, T. J. & Siuzdak, G. Antiviral agent blocks breathing of the common cold virus. Proc. Natl Acad. Sci. USA 95, 6774–6778 (1998).
Dowd, K. A., Jost, C. A., Durbin, A. P., Whitehead, S. S. & Pierson, T. C. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. 7, e1002111 (2011).
Kuhn, R. J., Dowd, K. A., Beth Post, C. & Pierson, T. C. Shake, rattle, and roll: impact of the dynamics of flavivirus particles on their interactions with the host. Virology 479–480, 508–517 (2015).
Moore, J. P. & Sodroski, J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70, 1863–1872 (1996).
Acharya, P. et al. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J. Virol. 88, 12895–12906 (2014).
Pincus, S. H. et al. In vivo efficacy of anti-glycoprotein 41, but not anti-glycoprotein 120, immunotoxins in a mouse model of HIV infection. J. Immunol. 170, 2236–2241 (2003).
Craig, R. B., Summa, C. M., Corti, M. & Pincus, S. H. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS ONE 7, e46778 (2012).
Santra, S. et al. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog. 11, e1005042 (2015).
Finnegan, C. M., Berg, W., Lewis, G. K. & DeVico, A. L. Antigenic properties of the human immunodeficiency virus envelope during cell–cell fusion. J. Virol. 75, 11096–11105 (2001).
Ferrari, G. et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 85, 7029–7036 (2011).
Veillette, M. et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J. Virol. 88, 2633–2644 (2014).
Haynes, B. F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286 (2012).
Baum, L. L. et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 157, 2168–2173 (1996).
Forthal, D. N. et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 180, 1338–1341 (1999).
Lambotte, O. et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE 8, e74855 (2013).
Binley, J. M. et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74, 627–643 (2000).
Sung, J. A. M. et al. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Invest. 125, 4077–4090 (2015).
Hessell, A. J. et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84, 1302–1313 (2010).
Mascola, J. R. et al. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71, 7198–7206 (1997).
Mascola, J. R. et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73, 4009–4018 (1999).
Hofmann-Lehmann, R. et al. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75, 7470–7480 (2001).
Hofmann-Lehmann, R. et al. Postnatal pre- and postexposure passive immunization strategies: protection of neonatal macaques against oral simian–human immunodeficiency virus challenge. J. Med. Primatol. 31, 109–119 (2002).
Burton, D. R. et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl Acad. Sci. USA 108, 11181–11186 (2011).
Pegu, A. et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 6, 243ra88 (2014).
Sholukh, A. M. et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine 33, 2086–2095 (2015).
Hessell, A. J. et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5, e1000433 (2009).
Hessell, A. J. et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–104 (2007).
Hessell, A. J. et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15, 951–954 (2009).
Moldt, B. et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 86, 6189–6196 (2012).
Moldt, B. et al. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcγ receptors to define the role of effector functions in protection against HIV. J. Virol. 85, 10572–10581 (2011).
Moldt, B. et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl Acad. Sci. USA 109, 18921–18925 (2012).
Moog, C. et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 7, 46–56 (2013).
Lu, C. L. et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352, 1001–1004 (2016).
Shingai, M. et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503, 277–280 (2013).
Barouch, D. H. et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228 (2013).
Shingai, M. et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 211, 2061–2074 (2014).
Bolton, D. L. et al. Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J. Virol. 90, 1321–1332 (2016).
Hessell, A. J. et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med. 22, 362–368 (2016).
Bournazos, S. et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158, 1243–1253 (2014).
Caskey, M. et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015).
Schoofs, T. et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. 352, 997–1001 (2016).
Lynch, R. M. et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med. 7, 319ra206 (2015).
Scheid, J. F. et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560 (2016).
Gleason, M. K. et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Ther. 11, 2674–2684 (2012).
Vallera, D. A. et al. IL-15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res. 22, 3440–3450 (2016).
Kontermann, R. E. & Brinkmann, U. Bispecific antibodies. Drug Discov. Today 20, 838–847 (2015).
Mabondzo, A. et al. Bispecific antibody targeting of human immunodeficiency virus type 1 (HIV-1) glycoprotein 41 to human macrophages through the Fc IgG receptor I mediates neutralizing effects in HIV-1 infection. J. Infect. Dis. 166, 93–99 (1992).
Mabondzo, A. et al. Antibody-dependent cellular cytotoxicity and neutralization of human immunodeficiency virus type 1 by high affinity cross-linking of gp41 to human macrophage Fc IgG receptor using bispecific antibody. J. Gen. Virol. 75, 1451–1456 (1994).
Yin, S., Okada, N. & Okada, H. Elimination of latently HIV-1-infected cells by lymphoblasts armed with bifunctional antibody. Microbiol. Immunol. 45, 101–108 (2001).
Sun, M. et al. Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36. J. Acquir. Immune Defic. Syndr. 66, 473–483 (2014).
Asokan, M. et al. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J. Virol. 89, 12501–12512 (2015).
Bird, R. E. & Walker, B. W. Single chain antibody variable regions. Trends Biotechnol. 9, 132–137 (1991).
Wong, R. et al. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica 98, 1930–1938 (2013).
Hoffmann, P. et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int. J. Cancer 115, 98–104 (2005).
Nagorsen, D. & Baeuerle, P. A. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp. Cell Res. 317, 1255–1260 (2011).
Przepiorka, D. et al. FDA approval: blinatumomab. Clin. Cancer Res. 21, 4035–4039 (2015).
Johnson, S. et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J. Mol. Biol. 399, 436–449 (2010).
Moore, P. A. et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117, 4542–4551 (2011).
Chichili, G. R. et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 7, 289ra82 (2015).
Root, A. et al. Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies 5, 6 (2016).
Sloan, D. D. et al. Targeting HIV reservoir in infected CD4 T cells by dual-affinity re-targeting molecules (DARTs) that bind HIV envelope and recruit cytotoxic T cells. PLoS Pathog. 11, e1005233 (2015).
McLellan, J. S. et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480, 336–344 (2011).
Pegu, A. et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat. Commun. 6, 8447 (2015).
Gardner, M. R. et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519, 87–91 (2015).
Sturdevant, C. B. et al. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 11, e1004720 (2015).
Bednar, M. M. et al. Compartmentalization, viral evolution, and viral latency of HIV in the CNS. Curr. HIV/AIDS Rep. 12, 262–271 (2015).
Finnefrock, A. C. et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J. Immunol. 182, 980–987 (2009).
Kaufmann, D. E. & Walker, B. D. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 182, 5891–5897 (2009).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Perelson, A. S., Neumann, A. U., Markowitz, M., Leonard, J. M. & Ho, D. D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271, 1582–1586 (1996).
Gulick, R. M. et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337, 734–739 (1997).
Perelson, A. S. et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387, 188–191 (1997).
Spivak, A. M. et al. Dynamic constraints on the second phase compartment of HIV-infected cells. AIDS Res. Hum. Retroviruses 27, 759–761 (2011).
Deeks, S. G. HIV: shock and kill. Nature 487, 439–440 (2012).
Acknowledgements
This Review was supported by US National Institutes of Health grants to the Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (UM1 AI100645), the Duke Center for AIDS Research (P30 AI64518), the Collaboratory of AIDS Researchers for Eradication (CARE) based at the University of North Carolina (U19 AI096113), and the University of North Carolina Center for AIDS Research (P30 AI50410). The authors thank J. Pollara, C.-H. Chen and E. Boritz for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
B.F.H., S.K. and J.L.N. have patents submitted on HIV antibodies for use as DARTS and as antibody therapeutics. G.F. and G.D.T. declare no competing interests.
Glossary
- Combined antiretroviral therapy
-
(cART). A HIV treatment regimen that uses a combination of drugs from two different classes of antiretrovirals that can act as inhibitors of at least two enzymes essential for the replicative cycle of HIV-1. The four major classes of antiretrovirals are nucleotide reverse transcriptase inhibitors, non-nucleotide reverse transcriptase inhibitors, protease inhibitors and integrase inhibitors.
- Virus set point
-
The level of viraemia reported as viral RNA copies per ml of plasma in a person infected with HIV. The viraemia level becomes relatively stable after the period of acute HIV infection, and usually remains stable until the onset of late-stage AIDS. The set point is thought to reflect the balance between the replicative capacity of the virus and partial control by the immune system.
- Elite controllers
-
Elite controllers are canonically defined as people in whom viraemia is below the limit of 50 copies per ml of plasma for three consecutive measurements performed over a period of 1 year while not on antiretroviral therapy. The ability of these individuals to control virus replication in the absence of therapeutic intervention is related to unique aspects of their humoral and/or cellular immune responses.
- Neutralizing antibody
-
An antibody that defends a cell from infectious pathogens or toxins by neutralizing their biological effects. In the case of HIV-1, neutralizing antibodies prevent the virus from entering target cells expressing the CD4 receptor and the co-receptor CC-chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4).
- Quantitative viral outgrowth assay
-
(QVOA). A standard assay used to estimate the frequency of latently infected cells in an HIV-infected individual. The assay requires isolation of resting memory CD4 T cells from the infected patient on antiretroviral therapy. The cells are stimulated and co-cultured with allogeneic CD4 T cells in multiple wells in a limiting dilution manner for a minimum period of 2 weeks. The frequency of replication-competent virus, or as infectious units per million (IUPM), is assessed by measuring the frequency of cultures expressing HIV-1 p24 antigen in the supernatant.
- CD4+ follicular helper T (TFH) cells
-
A subset of the CD4 T cell population specialized in providing help to B cells within secondary lymphoid tissues. TFH cells are identified according to the cell surface expression of several different molecules, including CXC-chemokine receptor 5 (CXCR5), programmed cell death protein 1 (PD1), SLAM-associated protein (SAP) and inducible T cell co-stimulator (ICOS). These cells can also produce interleukin-21 and express the transcription factor B cell lymphoma 6 (BCL-6). TFH cells are important for the formation of the germinal centre where they regulate the differentiation of B cells into plasma cells and memory B cells, thus regulating the antibody responses against pathogens.
- Viral latency
-
The ability to remain dormant within the host cell to establish a persistent occult infection. In the case of HIV-1, the viral genome is integrated into the DNA of the host cell as a provirus. Latency can be reversed, allowing viral RNA, protein and even virion production from the latently infected cell upon viral genome reactivation as a consequence of cellular signals. This latent HIV DNA genome can also be expanded during cellular replication in the absence of viral gene expression.
- Escape mutations
-
Mutations in the virus genome that encode modified gene products that will retain their function and preserve replication and assembly of infectious particles, but prevent recognition by immune responses directed against the parental gene product.
- Latency reversing agents
-
(LRAs). A relatively new class of agents used to selectively activate the provirus by interfering with the mechanisms reportedly identified to prevent HIV-1 expression in the latently infected cells or by promoting transcription of the provirus. Ideally, these agents should specifically stimulate virus reactivation but induce minimal activation of cellular subsets of the immune system.
- Epitopes
-
Portions of a molecule that are recognized by the immune system as non-self antigens and therefore capable of inducing antibody and/or cellular immune responses.
- Transmitted/founder
-
The transmitted/founder virus is a molecular definition of the virus whose genomic sequence is identified following acute infection as the earliest virus transmitted and is responsible for the productive clinical infection.
Rights and permissions
About this article
Cite this article
Ferrari, G., Haynes, B., Koenig, S. et al. Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. Nat Rev Drug Discov 15, 823–834 (2016). https://doi.org/10.1038/nrd.2016.173
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.173
This article is cited by
-
Peptide Assembly on the Membrane Determines the HIV-1 Inhibitory Activity of Dual-Targeting Fusion Inhibitor Peptides
Scientific Reports (2019)
-
New Strategies of ARV: the Road to Simplification
Current HIV/AIDS Reports (2018)