Key Points
-
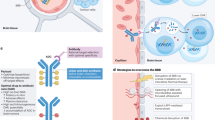
Cell-surface antigens expressed on glioblastoma cells that have proven resistant to signalling inhibition approaches — whether by tyrosine-kinase inhibitors or naked monoclonal antibodies — are attractive targets for antibody–drug conjugate (ADC) therapeutics
-
New-generation ADCs, exemplified by the newly developed tumour-selective anti-EGFR ADCs, are tolerable and have shown encouraging efficacy in early phase clinical trials
-
Emerging data from contemporary trials of ADCs can improve our understanding of the blood–brain barrier as a therapeutic hurdle in the treatment of patients with glioblastoma
-
Maximising the therapeutic potential of ADC-based therapies will require a paradigm shift in the approaches to investigating drug resistance, biomarker development, and functional imaging
-
ADCs can be used as monotherapies and, in addition, their limited toxicity profile can enable development of combinatorial approaches in rationally designed regimens to further enhance the therapeutic benefit of these agents
Abstract
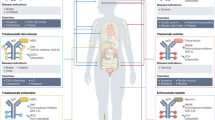
Glioblastomas are high-grade brain tumours with a poor prognosis and, currently, few available therapeutic options. This lack of effective treatments has been linked to diverse factors, including target selection, tumour heterogeneity and poor penetrance of therapeutic agents through the blood–brain barrier and into tumours. Therapies using monoclonal antibodies, alone or linked to cytotoxic payloads, have proved beneficial for patients with different solid tumours; these approaches are currently being explored in patients with glioblastoma. In this Review, we summarise clinical data regarding antibody–drug conjugates (ADCs) against a variety of targets in glioblastoma, and compare the efficacy and toxicity of targeting EGFR with ADCs versus naked antibodies in order to illustrate key aspects of the use of ADCs in this malignancy. Finally, we discuss the complex challenges related to the biology and mutational changes of glioblastoma that can affect the use of ADC-based therapies in patients with this disease, and highlight potential strategies to improve efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Chinot, O. L. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722 (2014).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Gilbert, M. R. et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J. Clin. Oncol. 31, 4085–4091 (2013).
Stupp, R. et al. Interim analysis of the EF-14 trial: a prospective, multi-center trial of NovoTTF-100A together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM [abstract NT-40]. Neuro Oncol. 16, v167 (2014).
Stupp, R. et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 314, 2535–2543 (2015).
Wong, E. T. et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J. Clin. Oncol. 17, 2572–2578 (1999).
van den Bent, M. J. et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 27, 1268–1274 (2009).
Batchelor, T. T. et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 31, 3212–3218 (2013).
Wick, W. et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 28, 1168–1174 (2010).
Taal, W. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 15, 943–953 (2014).
Gilbert, M. R. Antiangiogenic therapy for glioblastoma: complex biology and complicated results. J. Clin. Oncol. 34, 1567–1569 (2016).
Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Friedman, H. S. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740 (2009).
Kreisl, T. N. et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27, 740–745 (2009).
Wick, W. et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of glioblastoma: the EORTC 26101 trial [abstract LB-05]. Neuro Oncol. 17, v1 (2015).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Thorne, A. H., Zanca, C. & Furnari, F. Epidermal growth factor receptor targeting and challenges in glioblastoma. Neuro Oncol. 18, 914–918 (2016).
Gan, H. K., Kaye, A. H. & Luwor, R. B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 16, 748–754 (2009).
Liu, X. J. et al. A minority subpopulation of CD133+ /EGFRvIII+ /EGFR− cells acquires stemness and contributes to gefitinib resistance. CNS Neurosci. Ther. 19, 494–502 (2013).
Eimer, S. et al. Cyclopamine cooperates with EGFR inhibition to deplete stem-like cancer cells in glioblastoma-derived spheroid cultures. Neuro Oncol. 14, 1441–1451 (2012).
Emlet, D. R. et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 74, 1238–1249 (2014).
Westphal, M. et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer 51, 522–532 (2015).
Zhu, Y. & Shah, K. Multiple lesions in receptor tyrosine kinase pathway determine glioblastoma response to pan-ERBB inhibitor PF-00299804 and PI3K/mTOR dual inhibitor PF-05212384. Cancer Biol. Ther. 15, 815–822 (2014).
Clark, P. A. et al. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia 14, 420–428 (2012).
Akhavan, D. et al. De-repression of PDGFRbeta transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 3, 534–547 (2013).
Hegi, M. E. et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib — a phase II trial. Mol. Cancer Ther. 10, 1102–1112 (2011).
Wen, P. Y. et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02. Neuro Oncol. 16, 567–578 (2014).
Kreisl, T. N. et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J. Neurooncol. 92, 99–105 (2009).
Reardon, D. A. et al. Phase I study of AEE788, a novel multitarget inhibitor of ErbB- and VEGF-receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother. Pharmacol. 69, 1507–1518 (2012).
Pardridge, W. M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972 (2012).
Sampson, J. H. et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 10, 320–329 (2008).
Chandramohan, V., Sampson, J. H., Pastan, I. & Bigner, D. D. Toxin-based targeted therapy for malignant brain tumors. Clin. Dev. Immunol. 2012, 15 (2012).
Desjardins, A. et al. Phase I dose escalation study of D2C7-IT administered intratumorally via convection enhanced delivery (CED) for recurrent malignant glioma (MG) [abstract A42692]. Presented in Society of Neuro-Oncology Annual Meeting (2016).
Kunwar, S. et al. Phase III randomized trial of CED of IL13-PE38QQR versus Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 12, 871–881 (2010).
Jarboe, J. S., Johnson, K. R., Choi, Y., Lonser, R. R. & Park, J. K. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 67, 7983–7986 (2007).
Sampson, J. H. et al. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am. J. Roentgenol. 188, 703–709 (2007).
Patel, S. J. et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 56, 1243–1252 (2005).
Reardon, D. A. et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 10, 182–189 (2008).
Weaver, M. & Laske, D. W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 65, 3–13 (2003).
Tortorella, S. & Karagiannis, T. C. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J. Membr. Biol. 247, 291–307 (2014).
Weber, F. et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 64, 125–137 (2003).
Rand, R. W. et al. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin. Cancer Res. 6, 2157–2165 (2000).
Joshi, B. H. et al. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 61, 8058–8061 (2001).
Li, L. et al. A phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 113, 192–198 (2010).
Casaco, A. et al. Phase I single-dose study of intracavitary-administered nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol. Ther. 7, 333–339 (2008).
Reardon, D. A. et al. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 20, 1389–1397 (2002).
Riva, P. et al. Local treatment of malignant gliomas by direct infusion of specific monoclonal antibodies labeled with 131I: comparison of the results obtained in recurrent and newly diagnosed tumors. Cancer Res. 55, 5952s–5956s (1995).
Riva, P. et al. Loco-regional radioimmunotherapy of high-grade malignant gliomas using specific monoclonal antibodies labeled with 90Y: a phase I study. Clin. Cancer Res. 5, 3275s–3280s (1999).
Riva, P. et al. 131I radioconjugated antibodies for the locoregional radioimmunotherapy of high-grade malignant glioma — phase I and II study. Acta Oncol. 38, 351–359 (1999).
Merlo, A. et al. Biodistribution of 111In-labelled SCN-bz-DTPA-BC-2 MAb following loco-regional injection into glioblastomas. Int. J. Cancer 71, 810–816 (1997).
Bartolomei, M. et al. Combined treatment of glioblastoma patients with locoregional pre-targeted 90Y-biotin radioimmunotherapy and temozolomide. Q. J. Nucl. Med. Mol. Imaging 48, 220–228 (2004).
Paganelli, G. et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother. Radiopharm. 16, 227–235 (2001).
Goetz, C. et al. Locoregional radioimmunotherapy in selected patients with malignant glioma: experiences, side effects and survival times. J. Neurooncol. 62, 321–328 (2003).
Tsai, A. K. et al. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J. Neurooncol. 103, 255–266 (2011).
Cokgor, I. et al. Phase I trial results of iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 18, 3862–3872 (2000).
Zalutsky, M. R. et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 49, 30–38 (2008).
Riva, P. et al. Intralesional radioimmunotherapy of malignant gliomas. An effective treatment in recurrent tumors. Cancer 73, 1076–1082 (1994).
Riva, P. et al. Local application of radiolabeled monoclonal antibodies in the treatment of high grade malignant gliomas: a six-year clinical experience. Cancer 80, 2733–2742 (1997).
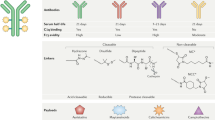
Parakh, S., Parslow, A. C., Gan, H. K. & Scott, A. M. Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin. Drug Deliv. 13, 401–419 (2016).
Faillot, T. et al. A phase I study of an anti-epidermal growth factor receptor monoclonal antibody for the treatment of malignant gliomas. Neurosurgery 39, 478–483 (1996).
Zhu, J., Takahashi, H. & Nakazawa, S. Human monoclonal antibody-drug conjugates in the experimental treatment of malignant gliomas — studies in vitro and in vivo. Neurol. Med. Chir. (Tokyo) 34, 279–285 (1994).
Hamblett, K. J. et al. AMG 595, an anti-EGFRvIII antibody-drug conjugate, induces potent antitumor activity against EGFRvIII-expressing glioblastoma. Mol. Cancer Ther. 14, 1614–1624 (2015).
Damore, M. A. et al. An EGFRvIII-specific IHC IUO test for patient selection in AMG 595 phase I trial [abstract]. J. Clin. Oncol. 31 (Suppl.), 2071 (2013).
Rosenthal, M. An antibody drug conjugate directed against the mutant receptor EGFRvIII for the treatment of glioblastoma [AMG 595]. Presented in AACR Annual Meeting (2014).
Van den Bent, M. et al. Efficacy of a novel antibody-drug conjugate (ADC), ABT-414, as monotherapy in epidermal growth factor receptor (EGFR) amplified, recurrent glioblastoma (GBM) [abstract]. J. Clin. Oncol. 34 (Suppl.), 2542 (2016).
Lassman, A. B. et al. Efficacy of a novel antibody-drug conjugate (ADC), ABT-414, with temozolomide (TMZ) in refractory glioblastoma (rGBM). Ann. Oncol. 27, 103–113 (2016).
Reardon, D. A. et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed patients with glioblastoma. Neuro Oncol. 19, 965–975 (2017).
Garrett, T. P. J. et al. Antibodies specifically targeting a locally misfolded region of tumor associated EGFR. Proc. Natl Acad. Sci. USA 106, 5082–5087 (2009).
Gan, H. K., Burgess, A. W., Clayton, A. H. A. & Scott, A. M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 72, 2924–2930 (2012).
Reilly, E. B. et al. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol. Cancer Ther. 14, 1141–1151 (2015).
Jungbluth, A. A. et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc. Natl Acad. Sci. USA 100, 639–644 (2003).
Scott, A. M. et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl Acad. Sci. USA 104, 4071–4076 (2007).
Gan, H. K. et al. A phase I and biodistribution study of ABT-806i, an 111indium-labeled conjugate of the tumor-specific anti-EGFR antibody ABT-806. J. Clin. Oncol. 31, A2520 (2013).
Gan, H. K. et al. ABT-414 mono- or combination therapy with temozolomide (TMZ) re-challenge in patients with recurrent glioblastoma (GBM) and amplified epidermal growth factor receptor (EGFR): a phase 1 study [abstract ATNT-01]. Presented in Society of Neuro-Oncology Annual Meeting (2015).
Mascai, M. et al. Corneal toxicity of ABT-414 in glioblastoma (GBM): clinical manifestation, ophthalmological findings and management [abstract A269]. Presented in Association for Research in Vision and Opthalmology (2016).
Thompson, J. A. et al. Phase I studies of anti-ENPP3 antibody drug conjugates (ADCs) in advanced refractory renal cell carcinomas (RRCC) [abstract]. J. Clin. Oncol. 33, 2503 (2015).
Ott, P. A. et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J. Clin. Oncol. 32, 3659–3666 (2014).
Ogura, M. et al. Phase I / II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30-positive Hodgkin's lymphoma or systemic anaplastic large-cell lymphoma. Cancer Sci. 105, 840–846 (2014).
Chandramohan, V. et al. Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin. Cancer Res. 19, 4717–4727 (2013).
Luther, N. et al. Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38. Mol. Cancer Ther. 9, 1039–1046 (2010).
Kuan, C. T. et al. Affinity-matured anti-glycoprotein NMB recombinant immunotoxins targeting malignant gliomas and melanomas. Int. J. Cancer 129, 111–121 (2011).
Foehr, E. D. et al. Targeting of the receptor protein tyrosine phosphatase beta with a monoclonal antibody delays tumor growth in a glioblastoma model. Cancer Res. 66, 2271–2278 (2006).
Wild, R., Dhanabal, M., Olson, T. A. & Ramakrishnan, S. Inhibition of angiogenesis and tumour growth by VEGF121-toxin conjugate: differential effect on proliferating endothelial cells. Br. J. Cancer 83, 1077–1083 (2000).
Gilabert-Oriol, R. et al. Dianthin-30 or gelonin versus monomethyl auristatin E, each configured with an anti-calcitonin receptor antibody, are differentially potent in vitro in high-grade glioma cell lines derived from glioblastoma. Cancer Immunol. Immunother. http://dx.doi.org/10.1007/s00262-017-2013-z (2017).
Meng, J. et al. A bivalent recombinant immunotoxin with high potency against tumors with EGFR and EGFRvIII expression. Cancer Biol. Ther. 16, 1764–774 (2015).
Nielsen, C. F. et al. The collagen receptor uPARAP/Endo180 as a novel target for antibody-drug conjugate mediated treatment of mesenchymal and leukemic cancers. Oncotarget http://dx.doi.org/10.18632/oncotarget.17883 (2017).
Kato, Y. et al. Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl. Med. Biol. 37, 785–794 (2010).
Hens, M., Vaidyanathan, G., Zhao, X. G., Bigner, D. D. & Zalutsky, M. R. Anti-EGFRvIII monoclonal antibody armed with 177Lu: in vivo comparison of macrocyclic and acyclic ligands. Nucl. Med. Biol. 37, 741–750 (2010).
Veeravagu, A. et al. Integrin alphavbeta3-targeted radioimmunotherapy of glioblastoma multiforme. Clin. Cancer Res. 14, 7330–7339 (2008).
Rustamzadeh, E. et al. Immunotoxin pharmacokinetics: a comparison of the anti-glioblastoma bi-specific fusion protein (DTAT13) to DTAT and DTIL13. J. Neurooncol. 77, 257–266 (2006).
Stish, B. J., Oh, S. & Vallera, D. A. Anti-glioblastoma effect of a recombinant bispecific cytotoxin cotargeting human IL-13 and EGF receptors in a mouse xenograft model. J. Neurooncol. 87, 51–61 (2008).
Todhunter, D. A. et al. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng. Des. Sel. 17, 157–164 (2004).
Oh, S., Tsai, A. K., Ohlfest, J. R., Panoskaltsis-Mortari, A. & Vallera, D. A. Evaluation of a bispecific biological drug designed to simultaneously target glioblastoma and its neovasculature in the brain. J. Neurosurg. 114, 1662–1671 (2011).
Zalutsky, M. R., Moseley, R. P., Coakham, H. B., Coleman, R. E. & Bigner, D. D. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 49, 2807–2813 (1989).
Kalofonos, H. P. et al. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J. Nucl. Med. 30, 1636–1645 (1989).
Zalutsky, M. R. et al. Monoclonal antibody and F(ab')2 fragment delivery to tumor in patients with glioma: comparison of intracarotid and intravenous administration. Cancer Res. 50, 4105–4110 (1990).
Zalutsky, M. R., Archer, G. E., Garg, P. K., Batra, S. K. & Bigner, D. D. Chimeric anti-tenascin antibody 81C6: increased tumor localization compared with its murine parent. Nucl. Med. Biol. 23, 449–458 (1996).
DiPippo, V. A. et al. Efficacy studies of an antibody-drug conjugate PSMA-ADC in patient-derived prostate cancer xenografts. Prostate 75, 303–313 (2015).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Francis, J. M. et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 4, 956–971 (2014).
Van den Bent, M. Identifying the correct patient (pt) population for ABT-414: Biomarker assays for epidermal growth factor receptor (EGFR) in pts with glioblastoma (GBM) [abstract 2903]. Presented in European Cancer Congress, (2015).
van den Bent, M. J. et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 17, 935–941 (2015).
Weller, M. et al. An international, double-blind, phase 3 trial of rindopepimut in newly diagnosed, EGFRvIII-expressing glioblastoma [abstract ATIM-13]. Presented in Society of Neuro-Oncology Annual Meeting (2016).
Xiu, J. et al. Tumor profiling on 1245 gliomas and paired tumor study on 19 high grade gliomas [abstract]. J. Clin. Oncol. 33, 2058 (2015).
Bozinov, O. et al. Decreasing expression of the interleukin-13 receptor IL-13Ralpha2 in treated recurrent malignant gliomas. Neurol. Med. Chir. (Tokyo) 50, 617–621 (2010).
Gensler, S. et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 5, e1119354 (2016).
Weickhardt, A. J. et al. Dual targeting of the epidermal growth factor receptor (EGFR) using the combination of cetuximab and erlotinib: pre-clinical evaluation and results of the phase II DUX study in chemotherapy refractory, advanced colorectal cancer. J. Clin. Oncol. 30, 1505–1512 (2011).
Gan, H. K. et al. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J. Biol. Chem. 282, 2840–2850 (2007).
Scott, A. M. et al. Determinants of response and resistance to ABT-414: results of next generation sequencing [abstract ATNT-02]. Neuro Oncol. 17, v10 (2015).
Sun, J. et al. A pilot study on EGFR-targeted molecular imaging of PET/CT With 11C-PD153035 in human gliomas. Clin. Nucl. Med. 39, e20–e26 (2014).
Ciprotti, M. et al. Phase I imaging and pharmacodynamic trial of CS-1008 in patients with metastatic colorectal cancer. J. Clin. Oncol. 33, 2609–2616 (2015).
Qin, D. X., Zheng, R., Tang, J., Li, J. X. & Hu, Y. H. Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 19, 1507–1510 (1990).
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Higgins, S. C., Fillmore, H. L., Ashkan, K., Butt, A. M. & Pilkington, G. J. Dual targeting NG2 and GD3A using Mab-Zap immunotoxin results in reduced glioma cell viability in vitro. Anticancer Res. 35, 77–84 (2015).
Gan, H. K. et al. A phase 1 study evaluating ABT-414 in combination with temozolomide (TMZ) for subjects with recurrent or unresectable glioblastoma (GBM) [abstract]. J. Clin. Oncol. 33, 2021 (2014).
Kawakami, K., Kawakami, M., Liu, Q. & Puri, R. K. Combined effects of radiation and interleukin-13 receptor-targeted cytotoxin on glioblastoma cell lines. Int. J. Radiat. Oncol. Biol. Phys. 63, 230–237 (2005).
Parakh, S. et al. Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin. Drug Deliv. 13, 401–419 (2016).
Brown, P. D. et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 26, 5603–5609 (2008).
Prados, M. D. et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J. Clin. Oncol. 27, 579–584 (2009).
Chakravarti, A. et al. RTOG 0211: a phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiat. Oncol. Biol. Phys. 85, 1206–1211 (2013).
Uhm, J. H. et al. Phase II evaluation of gefitinib in patients with newly diagnosed grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 80, 347–353 (2011).
Raizer, J. J. et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 12, 95–103 (2010).
Yung, W. K. et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 12, 1061–1070 (2010).
Franceschi, E. et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br. J. Cancer 96, 1047–1051 (2007).
Rich, J. N. et al. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 22, 133–142 (2004).
Neyns, B. et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol. 20, 1596–1603 (2009).
Lv, S. et al. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int. J. Oncol. 41, 1029–1035 (2012).
Lassman, A. et al. A phase 1 study evaluating ABT-414 with temozolomide (TMZ) or concurrent radiotherapy (RT) and TMZ in glioblastoma (GBM) [abstract]. Neurology 84, S43.006 (2015).
Acknowledgements
The authors were supported by the Cure Brain Cancer Foundation, the Operational Infrastructure Support Program, funding provided by the Victorian Government, the NHMRC Project Grant 1087580 and the NHMRC Program grant 1092788. H.K.G. is a recipient of a VCA Clinical Research Fellowship. A.M.S. is a recipient of an NHMRC Senior Practitioner Fellowship
Author information
Authors and Affiliations
Contributions
H.K.G. and A.M.S. wrote the manuscript. H.K.G., M.vD.B., D.A.R. edited the manuscript, and all authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.K.G. is conducting an investigator-initiated study with AbbVie; has received travel support and research funding from AbbVie and Ignyta; honoraria from AbbVie, BMS, Merck Serono and Pfizer; and is affiliated with the Ludwig Institute for Cancer Research (New York, New York, USA). M.vD.B. has received honoraria from AbbVie, Actelion, Blue Earth Diagnostics, BMS, Cavion, Celldex, Merck, Novocure and Roche; and research funding from AbbVie and Roche. A.B.L. has received honoraria from Bioclinica, Cortice Biosciences, Genentech, Oxigene, prIME Oncology, Sapience Therapeutics and VBI Vaccines. D.A.R. has received honoraria from and has a consulting or advisory role with AbbVie, Amgen, BMS, Cavion, Celldex, Genentech, Inovio, Juno Pharmaceuticals, Merck, Novartis, Novocure, Oxigene, Regeneron, Roche and Stemline Therapeutics; is involved in speakers' bureaus with Merck and Roche; and has received research funding from Celldex, Incyte and Midatech. A.M.S. owns stock in and has an advisory role with Life Science Pharmaceuticals; received research funding from AbbVie, Avipep and Daiichi Sankyo; has patents, royalties, or other intellectual property with Abbvie, Kalobios, Life Science Pharmaceuticals, and Ludwig Institute for Cancer Research.
Rights and permissions
About this article
Cite this article
Gan, H., van den Bent, M., Lassman, A. et al. Antibody–drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat Rev Clin Oncol 14, 695–707 (2017). https://doi.org/10.1038/nrclinonc.2017.95
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.95
This article is cited by
-
Clinical Trials in the Brain Tumour Population: Challenges and Strategies for the Future
Current Oncology Reports (2023)
-
Overcoming the blood–brain barrier for the therapy of malignant brain tumor: current status and prospects of drug delivery approaches
Journal of Nanobiotechnology (2022)
-
Multifunctional lipidic nanocarriers for effective therapy of glioblastoma: recent advances in stimuli-responsive, receptor and subcellular targeted approaches
Journal of Pharmaceutical Investigation (2022)
-
Excellent effects and possible mechanisms of action of a new antibody–drug conjugate against EGFR-positive triple-negative breast cancer
Military Medical Research (2021)
-
Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma
Nature Biomedical Engineering (2021)