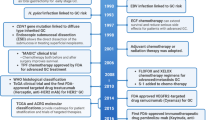
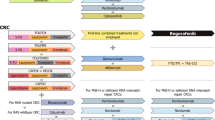
Key Points
-
Aberrant c-MET pathway activation occurs frequently in gastrointestinal tumours and can result from multiple mechanisms, including c-MET protein overexpression, MET amplification or enhanced transcription and/or aberrant autocrine or paracrine secretion of HGF
-
Activated c-MET signalling results in enhanced cancer cell proliferation, survival and invasion; furthermore, a complex network of signalling involving other receptors enhances the potency and endurance of c-MET downstream signalling
-
Elevated c-MET expression/amplification has been associated with a poor clinical outcome in patients with gastro-oesophageal tumours, although conflicting reports exist with respect to a prognostic role of c-MET in colorectal cancer
-
Structural studies of HGF and c-MET have yielded important results that paved the way for the development of anti-HGF and anti-c-MET monoclonal antibodies and specific or nonspecific c-MET tyrosine kinase inhibitors
-
In contrast to the initial phase II studies, the phase III trials failed to show any clinical benefit from anti-HGF or anti-c-MET therapies in gastrointestinal tumours, even in patients with c-MET-positive disease
-
Additional biomarkers should be sought, using techniques such as MET RNA in situ hybridization (ISH) and MET single/double silver ISH and 'omics'-based approaches to identify patients that are likely to derive maximal benefits from anti-HGF/c-MET therapies
Abstract
Data from many preclinical studies, including those using cellular models of colorectal, gastric, gastro-oesophageal and gastro-oesophageal junction cancers, indicate that the hepatocyte growth factor (HGF)–hepatocyte growth factor receptor (c-MET) pathway is vital for the growth, survival and invasive potential of gastrointestinal cancers. Following the availability of data from these various studies, and data on c-MET expression as a biomarker that indicates a poor prognosis in patients with gastrointestinal cancer and increased c-MET expression, inhibitors targeting this pathway have entered the clinic in the past decade. However, the design of clinical trials that incorporate the use of HGF/c-MET inhibitors in their most appropriate genetic and molecular context remains crucial. Recognizing and responding to this challenge, the European Commission funded Framework 7 MErCuRIC programme is running a biomarker-enriched clinical trial investigating the efficacy of combined c-MET/MEK inhibition in patients with RAS-mutant or RAS-wild-type metastatic colorectal cancer with aberrant c-MET expression. The design of this trial enables the continued refinement of the predictive biomarker and co-development of companion diagnostics. In this Review, we focus on advances in our understanding of inhibition of the HGF/c-MET pathway in patients with gastro-intestinal cancers, the prominent challenges facing the clinical translation and implementation of agents targeting HGF/c-MET, and discuss the various efforts, and associated obstacles to the discovery and validation of biomarkers that will enable patient stratification in this context.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Van Schaeybroeck, S., Allen, W. L., Turkington, R. C. & Johnston, P. G. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat. Rev. Clin. Oncol. 8, 222–232 (2011).
Gomez-Martin, C. et al. A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond. Cancer Lett. 351, 30–40 (2014).
Bardelli, A. et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 3, 658–673 (2013).
Van Schaeybroeck, S. et al. ADAM17-dependent c-MET–STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep. 7, 1940–1955 (2014).
Takeuchi, H. et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin. Cancer Res. 9, 1480–1488 (2003).
Blumenschein, G. R. Jr, Mills, G. B. & Gonzalez-Angulo, A. M. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J. Clin. Oncol. 30, 3287–3296 (2012).
Cooper, C. S. et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311, 29–33 (1984).
Stoker, M., Gherardi, E., Perryman, M. & Gray, J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327, 239–242 (1987).
Nakamura, T. et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 342, 440–443 (1989).
Bottaro, D. P. et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251, 802–804 (1991).
Schmidt, C. et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373, 699–702 (1995).
Chmielowiec, J. et al. c-Met is essential for wound healing in the skin. J. Cell Biol. 177, 151–162 (2007).
Borowiak, M. et al. Met provides essential signals for liver regeneration. Proc. Natl Acad. Sci. USA 101, 10608–10613 (2004).
Boccaccio, C. & Comoglio, P. M. MET, a driver of invasive growth and cancer clonal evolution under therapeutic pressure. Curr. Opin. Cell Biol. 31, 98–105 (2014).
Di Renzo, M. F. et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin. Cancer Res. 1, 147–154 (1995).
Kwak, E. L. et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 5, 1271–1281 (2015).
El-Deiry, W. S. et al. Molecular profiling of 6,892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 16, 1726–1737 (2015).
De Oliveira, A. T. et al. MET Is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res. 29, 4807–4811 (2009).
Liu, Y., Yu, X. F., Zou, J. & Luo, Z. H. Prognostic value of c-Met in colorectal cancer: a meta-analysis. World J. Gastroenterol. 21, 3706–3710 (2015).
Peng, Z. et al. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS ONE 9, e84502 (2014).
Resnick, M. B., Routhier, J., Konkin, T., Sabo, E. & Pricolo, V. E. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin. Cancer Res. 10, 3069–3075 (2004).
Koeppen, H. et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib+/−onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin. Cancer Res. 20, 4488–4498 (2014).
Cunningham, D. et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study [abstract]. J. Clin. Oncol. 33 (Suppl.), 4000 (2015).
Shah, M. A. et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. http://dx.doi.org/10.1001/jamaoncol.2016.5580 (2016).
Iveson, T. et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 15, 1007–1018 (2014).
Doi, T. et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma:the RILOMET-2 trial [abstract]. J. Clin. Oncol. 33 (Suppl. 3), TPS226 (2015).
Van Schaeybroeck, S. et al. MErCuRIC1: a phase I study of MEK1/2 inhibitor PD-0325901 with cMET inhibitor crizotinib in RASMT and RASWT (with aberrant c-MET) metastatic colorectal cancer (mCRC) patients [abstract]. J. Clin. Oncol. 33 (Suppl.), TPS3632 (2015).
Komada, M. et al. Proteolytic processing of the hepatocyte growth factor/scatter factor receptor by furin. FEBS Lett. 328, 25–29 (1993).
Gherardi, E. et al. Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc. Natl Acad. Sci. USA 100, 12039–12044 (2003).
Lokker, N. A. et al. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 11, 2503–2510 (1992).
Andermarcher, E., Surani, M. A. & Gherardi, E. Co-expression of the HGF/SF and c-met genes during early mouse embryogenesis precedes reciprocal expression in adjacent tissues during organogenesis. Dev. Genet. 18, 254–266 (1996).
Holmes, O. et al. Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J. Mol. Biol. 367, 395–408 (2007).
Gherardi, E. et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc. Natl Acad. Sci. USA 103, 4046–4051 (2006).
Pelicci, G. et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene 10, 1631–1638 (1995).
Fixman, E. D., Fournier, T. M., Kamikura, D. M., Naujokas, M. A. & Park, M. Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J. Biol. Chem. 271, 13116–13122 (1996).
Zhang, Y. W., Wang, L. M., Jove, R. & Vande Woude, G. F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21, 217–226 (2002).
Sipeki, S. et al. Phosphatidylinositol 3-kinase contributes to Erk1/Erk2 MAP kinase activation associated with hepatocyte growth factor-induced cell scattering. Cell Signal. 11, 885–890 (1999).
Maroun, C. R. et al. The Gab1 PH domain is required for localization of Gab1 at sites of cell–cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 19, 1784–1799 (1999).
Maroun, C. R., Naujokas, M. A., Holgado-Madruga, M., Wong, A. J. & Park, M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 20, 8513–8525 (2000).
Fan, S. et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 24, 1749–1766 (2005).
Rahimi, N., Hung, W., Tremblay, E., Saulnier, R. & Elliott, B. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J. Biol. Chem. 273, 33714–33721 (1998).
Royal, I., Lamarche-Vane, N., Lamorte, L., Kaibuchi, K. & Park, M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 11, 1709–1725 (2000).
Lock, L. S., Royal, I., Naujokas, M. A. & Park, M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 275, 31536–31545 (2000).
Schaeper, U. et al. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149, 1419–1432 (2000).
Weidner, K. M. et al. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384, 173–176 (1996).
Garcia-Guzman, M., Dolfi, F., Zeh, K. & Vuori, K. Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1–Crk signaling complex formation. Oncogene 18, 7775–7786 (1999).
Gual, P. et al. Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene 19, 1509–1518 (2000).
Montagner, A. et al. A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation. J. Biol. Chem. 280, 5350–5360 (2005).
Orian-Rousseau, V., Chen, L., Sleeman, J. P., Herrlich, P. & Ponta, H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 16, 3074–3086 (2002).
Orian-Rousseau, V. et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol. Biol. Cell 18, 76–83 (2007).
Conrotto, P., Corso, S., Gamberini, S., Comoglio, P. M. & Giordano, S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene 23, 5131–5137 (2004).
Conrotto, P. et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 105, 4321–4329 (2005).
Giordano, S. et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat. Cell Biol. 4, 720–724 (2002).
Bertotti, A., Comoglio, P. M. & Trusolino, L. Beta4 integrin activates a Shp2–Src signaling pathway that sustains HGF-induced anchorage-independent growth. J. Cell Biol. 175, 993–1003 (2006).
Trusolino, L., Bertotti, A. & Comoglio, P. M. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107, 643–654 (2001).
Carter, S., Urbe, S. & Clague, M. J. The met receptor degradation pathway: requirement for Lys48-linked polyubiquitin independent of proteasome activity. J. Biol. Chem. 279, 52835–52839 (2004).
Peschard, P. et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8, 995–1004 (2001).
Hammond, D. E., Urbe, S., Vande Woude, G. F. & Clague, M. J. Down-regulation of MET, the receptor for hepatocyte growth factor. Oncogene 20, 2761–2770 (2001).
Petrelli, A. et al. The endophilin–CIN85–Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416, 187–190 (2002).
Kermorgant, S. & Parker, P. J. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J. Cell Biol. 182, 855–863 (2008).
Nath, D., Williamson, N. J., Jarvis, R. & Murphy, G. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J. Cell Sci. 114, 1213–1220 (2001).
Foveau, B. et al. Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis. Mol. Biol. Cell 20, 2495–2507 (2009).
Michieli, P. et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 6, 61–73 (2004).
Lee, J. H. et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 19, 4947–4953 (2000).
Ma, P. C. et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 63, 6272–6281 (2003).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Frampton, G. M. et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 5, 850–859 (2015).
Dulak, A. M. et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 45, 478–486 (2013).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Lennerz, J. K. et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J. Clin. Oncol. 29, 4803–4810 (2011).
Kammula, U. S. et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 248, 219–228 (2007).
Toiyama, Y. et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int. J. Cancer 130, 2912–2921 (2012).
Park, W. S. et al. Absence of mutations in the kinase domain of the Met gene and frequent expression of Met and HGF/SF protein in primary gastric carcinomas. APMIS 108, 195–200 (2000).
Zhao, J., Zhang, X. & Xin, Y. Up-regulated expression of Ezrin and c-Met proteins are related to the metastasis and prognosis of gastric carcinomas. Histol. Histopathol. 26, 1111–1120 (2011).
Fischer, O. M., Giordano, S., Comoglio, P. M. & Ullrich, A. Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J. Biol. Chem. 279, 28970–28978 (2004).
Jo, M. et al. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J. Biol. Chem. 275, 8806–8811 (2000).
Khoury, H. et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol. Biol. Cell 16, 550–561 (2005).
Bauer, T. W. et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol. Cancer Ther. 5, 1676–1682 (2006).
Salian-Mehta, S., Xu, M. & Wierman, M. E. AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Mol. Cell. Endocrinol. 374, 92–100 (2013).
Rong, S., Segal, S., Anver, M., Resau, J. H. & Vande Woude, G. F. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc. Natl Acad. Sci. USA 91, 4731–4735 (1994).
Moshitch-Moshkovitz, S. et al. In vivo direct molecular imaging of early tumorigenesis and malignant progression induced by transgenic expression of GFP-Met. Neoplasia 8, 353–363 (2006).
Giordano, S. et al. A point mutation in the MET oncogene abrogates metastasis without affecting transformation. Proc. Natl Acad. Sci. USA 94, 13868–13872 (1997).
Taniguchi, K. et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer 82, 2112–2122 (1998).
Wu, X. et al. Hepatocyte growth factor activates tumor stromal fibroblasts to promote tumorigenesis in gastric cancer. Cancer Lett. 335, 128–135 (2013).
Amemiya, H. et al. c-Met expression in gastric cancer with liver metastasis. Oncology 63, 286–296 (2002).
Bradley, C. A. et al. Transcriptional upregulation of c-MET is associated with invasion and tumor budding in colorectal cancer. Oncotarget 7, 78921–78945 (2016).
Jiang, W. G., Lloyds, D., Puntis, M. C., Nakamura, T. & Hallett, M. B. Regulation of spreading and growth of colon cancer cells by hepatocyte growth factor. Clin. Exp. Metastasis 11, 235–242 (1993).
Sun, Y. L. et al. Expression of HGF and Met in human tissues of colorectal cancers: biological and clinical implications for synchronous liver metastasis. Int. J. Med. Sci. 10, 548–559 (2013).
Zeng, Z. S. et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 265, 258–269 (2008).
Gayyed, M. F., Abd El-Maqsoud, N. M., El- Hameed El-Heeny, A. A. & Mohammed, M. F. c-MET expression in colorectal adenomas and primary carcinomas with its corresponding metastases. J. Gastrointest. Oncol. 6, 618–627 (2015).
Zou, H. Y. et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 67, 4408–4417 (2007).
Lee, H. E. et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br. J. Cancer 107, 325–333 (2012).
Nakajima, M. et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 85, 1894–1902 (1999).
Xu, Y. et al. Expression and clinical significance of c-Met in advanced esophageal squamous cell carcinoma. BMC Cancer 15, 6 (2015).
Fuse, N. et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer 19, 183–191 (2016).
Schweiger, T. et al. Clinical impact of c-MET expression and mutational status in patients with colorectal cancer lung metastases. Eur. J. Cardiothorac. Surg. 49, 1103–1111 (2016).
Al-Maghrabi, J. et al. c-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer 15, 676 (2015).
Voutsina, A. et al. Combined analysis of KRAS and PIK3CA mutations, MET and PTEN expression in primary tumors and corresponding metastases in colorectal cancer. Mod. Pathol. 26, 302–313 (2013).
Corso, S. & Giordano, S. Cell-autonomous and non-cell-autonomous mechanisms of HGF/MET-driven resistance to targeted therapies: from basic research to a clinical perspective. Cancer Discov. 3, 978–992 (2013).
Harbinski, F. et al. Rescue screens with secreted proteins reveal compensatory potential of receptor tyrosine kinases in driving cancer growth. CancerDiscov. 2, 948–959 (2012).
Chen, C. T. et al. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol. Cancer Ther. 11, 660–669 (2012).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124, 5145–5158 (2014).
Liska, D., Chen, C. T., Bachleitner-Hofmann, T., Christensen, J. G. & Weiser, M. R. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin. Cancer Res. 17, 472–482 (2011).
Pietrantonio, F. et al. MET-driven resistance to dual EGFR and BRAF blockade may be overcome by switching from EGFR to MET inhibition in BRAF-mutated colorectal cancer. Cancer Discov. 6, 963–971 (2016).
Diaz, L. A. Jr, Sausen, M., Fisher, G. A. & Velculescu, V. E. Insights into therapeutic resistance from whole-genome analyses of circulating tumor DNA. Oncotarget 4, 1856–1857 (2013).
Carson, R. et al. HDAC inhibition overcomes acute resistance to MEK inhibition in BRAF-mutant colorectal cancer by downregulation of c-FLIPL. Clin. Cancer Res. 21, 3230–3240 (2015).
De Bacco, F. et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J. Natl Cancer Inst. 103, 645–661 (2011).
Li, Y. et al. c-Met targeting enhances the effect of irradiation and chemical agents against malignant colon cells harboring a KRAS mutation. PLoS ONE 9, e113186 (2014).
Burgess, T. L. et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol. Cancer Ther. 9, 400–409 (2010).
Rosen, P. J. et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin. Cancer Res. 16, 2677–2687 (2010).
Schoffski, P. et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int. 108, 679–686 (2011).
Ryan, C. J. et al. Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin. Cancer Res. 19, 215–224 (2013).
Jones, S. F. et al. Safety, tolerability, and pharmacokinetics of TAK-701, a humanized anti-hepatocyte growth factor (HGF) monoclonal antibody, in patients with advanced nonhematologic malignancies: first-in-human phase I dose-escalation study [abstract]. J. Clin. Oncol. 28 (Suppl.), 3081 (2010).
Tabernero, J. et al. A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases. Clin. Cancer Res. 20, 2793–2804 (2014).
Merchant, M. et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl Acad. Sci. USA 110, E2987–E2996 (2013).
Spigel, D. R. et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 31, 4105–4114 (2013).
Spigel, D. R. et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial [abstract]. J. Clin. Oncol. 32 (Suppl.), 8000 (2014).
Petrelli, A. et al. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc. Natl Acad. Sci. USA 103, 5090–5095 (2006).
Schelter, F. et al. A disintegrin and metalloproteinase-10 (ADAM-10) mediates DN30 antibody-induced shedding of the met surface receptor. J. Biol. Chem. 285, 26335–26340 (2010).
Wang, J. et al. Anti-c-Met monoclonal antibody ABT-700 breaks oncogene addiction in tumors with MET amplification. BMC Cancer 16, 105 (2016).
Smith, M. R. et al. Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J. Clin. Oncol. 32, 3391–3399 (2014).
Choueiri, T. K. et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1814–1823 (2015).
Eder, J. P., Vande Woude, G. F., Boerner, S. A. & LoRusso, P. M. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 15, 2207–2214 (2009).
Eathiraj, S. et al. Discovery of a novel mode of protein kinase inhibition characterized by the mechanism of inhibition of human mesenchymal-epithelial transition factor (c-Met) protein autophosphorylation by ARQ 197. J. Biol. Chem. 286, 20666–20676 (2011).
Calles, A. et al. Tivantinib (ARQ 197) efficacy is independent of MET inhibition in non-small-cell lung cancer cell lines. Mol. Oncol. 9, 260–269 (2015).
Basilico, C. et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin. Cancer Res. 19, 2381–2392 (2013).
Malka, D. et al. FOLFOX alone or combined to rilotumumab or panitumumab as first-line treatment in patients (pts) with advanced gastroesophageal adenocarcinoma (AGEA): An open-label, randomized phase II trial (PRODIGE 17 ACCORD 20 MEGA) [abstract]. J. Clin. Oncol. 33 (Suppl.), 4013 (2015).
Van Cutsem, E. et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin. Cancer Res. 20, 4240–4250 (2014).
Shah, M. A. et al. Randomized phase II study of FOLFOX+/− MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC) [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 2 (2015).
Kang, Y. et al. Phase 1, open-label, dose-escalation, and expansion study of ABT-700, an anti-C-met antibody, in patients (pts) with advanced solid tumors [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 167 (2015).
Bendell, J. C. et al. A randomized, double-blind, phase II study of first-line FOLFOX plus bevacizumab with onartuzumab versus placebo in patients with metastatic colorectal cancer (mCRC) [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 663 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01472016 (2017).
Kwak, E. et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 1 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02344810 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02205398 (2017).
Jhawer, M. et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study [abstract]. J. Clin. Oncol. 27 (Suppl.), 4502 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02435108 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02008383 (2016).
Eng, C. et al. A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int. J. Cancer 139, 177–186 (2016).
Rimassa, L. et al. Phase II study of tivantinib (ARQ 197) in combination with cetuximab in EGFR inhibitor-resistant, MET-high, KRAS wild-type (KRASwt) metastatic colorectal cancer (mCRC) [abstract]. Ann. Oncol. 26 (Suppl. 4), 108–116 (2015).
Dziadziuszko, R. et al. Correlation between MET gene copy number by silver in situ hybridization and protein expression by immunohistochemistry in non-small cell lung cancer. J. Thorac. Oncol. 7, 340–347 (2012).
Wang, F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14, 22–29 (2012).
Choi, J. et al. Analysis of MET mRNA expression in gastric cancers using RNA in situ hybridization assay: its clinical implication and comparison with immunohistochemistry and silver in situ hybridization. PLoS ONE 9, e111658 (2014).
Paik, P. K. et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 5, 842–849 (2015).
Lee, J. et al. Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget 6, 28211–28222 (2015).
Cappuzzo, F. et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl Cancer Inst. 97, 643–655 (2005).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25, 118–145 (2007).
Camidge, D. R. et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 32 (Suppl.), 8001 (2014).
Sadanandam, A. et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 19, 619–625 (2013).
Qi, J. et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 71, 1081–1091 (2011).
Cepero, V. et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 70, 7580–7590 (2010).
Petti, C. et al. Truncated RAF kinases drive resistance to MET inhibition in MET-addicted cancer cells. Oncotarget 6, 221–233 (2015).
Bachleitner-Hofmann, T. et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol. Cancer Ther. 7, 3499–3508 (2008).
Kim, D. C. et al. Resistance to the c-Met inhibitor KRC-108 induces the epithelial transition of gastric cancer cells. Oncol. Lett. 11, 991–997 (2016).
Finisguerra, V. et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015).
Acknowledgements
This work was supported by MErCuRIC, funded by the European Commission's Framework Programme 7, under contract #602901.
Author information
Authors and Affiliations
Consortia
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
P.L.P. has received honoraria from Amgen, Astrazeneca Boerhinger–Ingelheim, Integragen, Merck–Serono, Roche and Sanofi. J.T. has served on the advisory boards of Amgen, Bayer, Boehringer–Ingelheim, Celgene, Chugai, Lilly, MSD, Merck–Serono, Novartis, Pfizer, Roche, Sanofi, Symphogen, Taiho, and Takeda. P.G.J. is the founder and holds shares in Almac diagnostics, Fusion and CV6 Therapeutics, and has served as an expert adviser/consultant of Pfizer and Chugai Pharmaceuticals. The other authors declare no competing interests.
Supplementary information
Supplementary information S1 (table)
HGF and cMET inhibitors currently in preclinical development. (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Bradley, C., Salto-Tellez, M., Laurent-Puig, P. et al. Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol 14, 562–576 (2017). https://doi.org/10.1038/nrclinonc.2017.40
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.40
This article is cited by
-
ASAP2 interrupts c-MET-CIN85 interaction to sustain HGF/c-MET-induced malignant potentials in hepatocellular carcinoma
Experimental Hematology & Oncology (2023)
-
Circular RNA encoded MET variant promotes glioblastoma tumorigenesis
Nature Communications (2023)
-
HSF4 promotes tumor progression of colorectal cancer by transactivating c-MET
Molecular and Cellular Biochemistry (2023)
-
Helicobacter pylori–activated fibroblasts as a silent partner in gastric cancer development
Cancer and Metastasis Reviews (2023)
-
HGF-mediated elevation of ETV1 facilitates hepatocellular carcinoma metastasis through upregulating PTK2 and c-MET
Journal of Experimental & Clinical Cancer Research (2022)