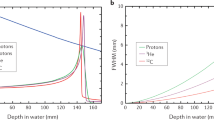
Key Points
-
Owing to their physical properties, the therapeutic use of charged particles in radiotherapy is advantageous over photon-based radiotherapy
-
The delivery of charged particles is more costly than that of X-rays, with no level 1 evidence currently indicating clinical superiority of either approach
-
Randomized trials are essential to establish the clinical benefit derived from charged-particle therapy; several studies are currently ongoing worldwide
-
The design of clinical trials for the comparison of different radiotherapy modalities is very complex; careful patient selection is essential to obtaining meaningful results
-
The criteria for patient selection for radiotherapy trials need to take dosimetric and radiobiological considerations into account
Abstract
Radiotherapy with high-energy charged particles has become an attractive therapeutic option for patients with several tumour types because this approach better spares healthy tissue from radiation than conventional photon therapy. The cost associated with the delivery of charged particles, however, is higher than that of even the most elaborate photon-delivery technologies. Reliable evidence of the relative cost-effectiveness of both modalities can only come from the results of randomized clinical trials. Thus, the hurdles that currently limit direct comparisons of these two approaches in clinical trials, especially those related to insurance coverage, should be removed. Herein, we review several randomized trials of charged-particle therapies that are ongoing, with results that will enable selective delivery to patients who are most likely to benefit from them. We also discuss aspects related to radiobiology, including the immune response and hypoxia, which will need to be taken into consideration in future randomized trials to fully exploit the potential of charged particles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thariat, J., Hannoun-Levi, J.-M., Sun Myint, A., Vuong, T. & Gérard, J.-P. Past, present, and future of radiotherapy for the benefit of patients. Nat. Rev. Clin. Oncol. 10, 52–60 (2012).
Jermann, M. Particle therapy statistics in 2014. Int. J. Part. Ther. 2, 50–54 (2015).
Durante, M. & Paganetti, H. Nuclear physics in particle therapy: a review. Rep. Prog. Phys. 79, 96702 (2016).
Kooy, H. M. & Grassberger, C. Intensity modulated proton therapy. Br. J. Radiol. 88, 20150195 (2015).
Bert, C. & Durante, M. Motion in radiotherapy: particle therapy. Phys. Med. Biol. 56, R113–R144 (2011).
Baumann, M. et al. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 16, 234–249 (2016).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 7, 37–43 (2010).
Lievens, Y. & Pijls-Johannesma, M. Health economic controversy and cost-effectiveness of proton therapy. Semin. Radiat. Oncol. 23, 134–141 (2013).
Paganetti, H. & Zietman, A. Why is proton beam therapy so controversial? J. Am. Coll. Radiol. 12, 1318–1319 (2015).
Loeffler, J. S. & Durante, M. Charged particle therapy — optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 10, 411–424 (2013).
Loeffler, J. S. Technology assessment in radiation oncology: time for reassessment? Nat. Clin. Pract. Oncol. 5, 299–299 (2008).
Suit, H. et al. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother. Oncol. 86, 148–153 (2008).
Sakurai, H., Robert Lee, W. & Orton, G. C. We do not need randomized clinical trials to demonstrate the superiority of proton therapy. Med. Phys. 39, 1685–1687 (2012).
Bentzen, S. M. Radiation oncology health technology assessment: the best is the enemy of the good. Nat. Clin. Pract. Oncol. 5, 563 (2008).
Jaffray, D. A. Image-guided radiotherapy: from current concept to future perspectives. Nat. Rev. Clin. Oncol. 9, 688–699 (2012).
Bentzen, S. M. Randomized controlled trials in health technology assessment: overkill or overdue? Radiother. Oncol. 86, 142–147 (2008).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Zagar, T. M., Cardinale, D. M. & Marks, L. B. Breast cancer therapy-associated cardiovascular disease. Nat. Rev. Clin. Oncol. 13, 172–184 (2016).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998 (2013).
Mailhot Vega, R. B. et al. Establishing cost-effective allocation of proton therapy for breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 95, 11–18 (2016).
Dagan, R., Ho, M. W., Rutenberg, M. S., Li, Z. & Mendenhall, N. P. Two-year outcomes of a prospective study of proton therapy for breast cancer regional nodal irradiation [abstract]. J. Clin. Oncol. 33 (Suppl.), 65 (2015).
Verma, V., Shah, C. & Mehta, M. P. Clinical outcomes and toxicity of proton radiotherapy for breast cancer. Clin. Breast Cancer 16, 145–154 (2016).
Akamatsu, H. et al. First experience of carbon-ion radiotherapy for early breast cancer. Jpn J. Radiol. 32, 288–295 (2014).
Martin, N. E. & D'Amico, A. V. Progress and controversies: radiation therapy for prostate cancer. CA Cancer J. Clin. 64, 389–407 (2014).
Zaorsky, N. G. et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 10, 565–579 (2013).
Wallis, C. J. D. et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur. Urol. 70, 21–30 (2015).
Roach, M., Ceron Lizarraga, T. L. & Lazar, A. A. Radical prostatectomy versus radiation and androgen deprivation therapy for clinically localized prostate cancer: how good is the evidence? Int. J. Radiat. Oncol. Biol. Phys. 93, 1064–1070 (2015).
Lennernäs, B. et al. Radical prostatectomy versus high-dose irradiation in localized/locally advanced prostate cancer: a Swedish multicenter randomized trial with patient-reported outcomes. Acta Oncol. 54, 875–881 (2015).
Yu, J. B. et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J. Natl Cancer Inst. 105, 25–32 (2013).
Sheets, N. C. et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307, 1611–1620 (2012).
Bryant, C. et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 95, 422–434 (2016).
Schiller, K. C., Habl, G. & Combs, S. E. Protons, photons, and the prostate — is there emerging evidence in the ongoing discussion on particle therapy for the treatment of prostate cancer? Front. Oncol. 6, 8 (2016).
Durante, M. Charged particles for liver cancer. Ann. Transl Med. 3, 2–5 (2015).
Hong, T. S. et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 34, 460–468 (2016).
Granovetter, M. Proton radiotherapy for primary liver cancers. Lancet Oncol. 17, e49 (2016).
Kamada, T. et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 16, e93–e100 (2015).
Gondi, V., Yock, T. I. & Mehta, M. P. Proton therapy for paediatric CNS tumours — improving treatment-related outcomes. Nat. Rev. Neurol. 12, 334–345 (2016).
Tanaka, S., Louis, D. N., Curry, W. T., Batchelor, T. T. & Dietrich, J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat. Rev. Clin. Oncol. 10, 14–26 (2013).
Malvezzi, M. et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann. Oncol. 27, 725–731 (2016).
Durante, M., Tommasino, F. & Yamada, S. Modeling combined chemotherapy and particle therapy for locally advanced pancreatic cancer. Front. Oncol. 5, 145 (2015).
Hammel, P. et al. Effect of chemoradiotherapy versus chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 315, 1844–1853 (2016).
Crane, C. H. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J. Radiat. Res. 57, i53–i57 (2016).
Chadha, A. S. et al. Phase I trial of consolidative radiotherapy with concurrent bevacizumab, erlotinib and capecitabine for unresectable pancreatic cancer. PLoS ONE 11, 1–15 (2016).
Krishnan, S. et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 94, 755–765 (2016).
Terashima, K. et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. 103, 25–31 (2012).
Shinoto, M. et al. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 95, 498–504 (2016).
Wang, X., Hu, C. & Eisbruch, A. Organ-sparing radiation therapy for head and neck cancer. Nat. Rev. Clin. Oncol. 8, 639–648 (2011).
Lukens, J. N., Lin, A. & Hahn, S. M. Proton therapy for head and neck cancer. Curr. Opin. Oncol. 27, 165–171 (2015).
Gregoire, V., Langendijk, J. A. & Nuyts, S. Advances in radiotherapy for head and neck cancer. J. Clin. Oncol. 33, 3277–3284 (2015).
Patel, S. H. et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 15, 1027–1038 (2014).
Jensen, A. D. et al. High-LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years' experience with raster-scanned carbon ion therapy. Radiother. Oncol. 118, 272–280 (2016).
Jensen, A. D. et al. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer 121, 3001–3009 (2015).
Lo, S. S. et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010).
Maquilan, G. & Timmerman, R. Stereotactic body radiation therapy for early-stage lung cancer. Cancer J. 22, 274–279 (2016).
Bertolaccini, L., Terzi, A., Ricchetti, F. & Alongi, F. Surgery or stereotactic ablative radiation therapy: how will be treated operable patients with early stage not small cell lung cancer in the next future? Ann. Transl Med. 3, 25 (2015).
De Ruysscher, D. & Chang, J. Y. Clinical controversies: proton therapy for thoracic tumors. Semin. Radiat. Oncol. 23, 115–119 (2013).
Berman, A., James, S. & Rengan, R. Proton beam therapy for non-small cell lung cancer: current clinical evidence and future directions. Cancers (Basel). 7, 1178–1190 (2015).
Mauguen, A. et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients' data. Lancet Oncol. 14, 619–626 (2013).
Machtay, M. et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 82, 425–434 (2012).
Bradley, J. D. et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16, 187–199 (2015).
Eaton, B. R. et al. Institutional enrollment and survival among NSCLC patients receiving chemoradiation: NRG Oncology Radiation Therapy Oncology Group (RTOG) 0617. J. Natl. Cancer Inst. 108, djw034 (2016).
Tucker, S. L. et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother. Oncol. 119, 495–500 (2016).
Hong, J. C. & Salama, J. K. Dose escalation for unresectable locally advanced non-small cell lung cancer: end of the line? Transl Lung Cancer Res. 5, 126–133 (2016).
Movsas, B. et al. Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: a secondary analysis of the Radiation Therapy Oncology Group 0617 randomized clinical trial. JAMA Oncol. 2, 359–367 (2016).
Roelofs, E. et al. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J. Thorac. Oncol. 7, 165–176 (2012).
Chang, J. Y. et al. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 95, 505–516 (2016).
Liao, Z. X. et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced nonsmall cell lung cancer [abstract]. J. Clin. Oncol. 34 (Suppl.), 8500 (2016).
Graeff, C., Lüchtenborg, R., Eley, J. G., Durante, M. & Bert, C. A. 4D-optimization concept for scanned ion beam therapy. Radiother. Oncol. 109, 419–424 (2013).
Riboldi, M., Orecchia, P. R. & Baroni, P. G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol. 13, e383–e391 (2012).
Graeff, C., Constantinescu, A., Luchtenborg, R., Durante, M. & Bert, C. Multigating, a 4D optimized beam tracking in scanned ion beam therapy. Technol. Cancer Res. Treat. 13, 497–504 (2014).
Wölfelschneider, J. et al. Impact of fractionation and number of fields on dose homogeneity for intra-fractionally moving lung tumors using scanned carbon ion treatment. Radiother. Oncol. 118, 498–503 (2016).
Anderle, K. et al. In silico comparison of photons versus carbon ions in single fraction therapy of lung cancer. Phys. Med. 32, 1118–1123 (2016).
Nikoghosyan, A. V. et al. Randomised trial of proton versus carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer 10, 607 (2010).
Mishra, K. K. et al. Long-term results of the UCSF-LBNL randomized trial: charged particle with helium ion versus iodine-125 plaque therapy for choroidal and ciliary body melanoma. Int. J. Radiat. Oncol. Biol. Phys. 92, 376–383 (2015).
Wedenberg, M. & Toma-Dasu, I. Disregarding RBE variation in treatment plan comparison may lead to bias in favor of proton plans. Med. Phys. 41, 91706 (2014).
Langendijk, J.A. et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother. Oncol. 107, 267–273 (2013).
Durante, M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 87, 20130626 (2014).
Schaue, D. & McBride, W. H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 12, 1–14 (2015).
Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 59, R419–R472 (2014).
Sabin, N. D. et al. Imaging changes in very young children with brain tumors treated with proton therapy and chemotherapy. Am. J. Neuroradiol. 34, 446–450 (2013).
Gunther, J. R. et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 93, 54–63 (2015).
Sethi, R. V. et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int. J. Radiat. Oncol. Biol. Phys. 88, 655–663 (2014).
Yock, T. I. et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 17, 287–298 (2016).
Peeler, C. R. et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother. Oncol. 121, 395–401 (2016).
Buchsbaum, J. C. et al. Range modulation in proton therapy planning: a simple method for mitigating effects of increased relative biological effectiveness at the end-of-range of clinical proton beams. Radiat. Oncol. 9, 2 (2014).
Fager, M. et al. Linear energy transfer painting with proton therapy: a means of reducing radiation doses with equivalent clinical effectiveness. Int. J. Radiat. Oncol. Biol. Phys. 91, 1057–1064 (2015).
Unkelbach, J., Botas, P., Giantsoudi, D., Gorissen, B. & Paganetti, H. Reoptimization of intensity-modulated proton therapy plans based on linear energy transfer. Int. J. Radiat. Oncol. Biol. Phys. 96, 1097–1106 (2016).
Castro, J. R. Results of heavy ion radiotherapy. Radiat. Environ. Biophys. 34, 45–48 (1995).
Castro, J. R. et al. Treatment of cancer with heavy charged particles. Int. J. Radiat. Oncol. Biol. Phys. 8, 2191–2198 (1982).
Pompos, A., Durante, M. & Choy, H. Heavy ions in cancer therapy. JAMA Oncol. 2, 1539–1540 (2016).
No authors listed. Report of the Cancer Moonshot Task Force. https://obamawhitehouse.archives.gov/sites/default/files/docs/final_cancer_moonshot_task_force_report_1.pdf (2016).
Grün, R. et al. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys. Med. Biol. 57, 7261–7274 (2012).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Strigari, L., Benassi, M., Sarnelli, A., Polico, R. & D'Andrea, M. A modified hypoxia-based TCP model to investigate the clinical outcome of stereotactic hypofractionated regimes for early stage non-small-cell lung cancer (NSCLC). Med. Phys. 39, 4502–4514 (2012).
Toma-Dasu, I., Sandström, H., Barsoum, P. & Dasu, A. To fractionate or not to fractionate? That is the question for the radiosurgery of hypoxic tumors. J. Neurosurg. 121 (Suppl.), 110–115 (2014).
McKeown, S. R. Defining normoxia physoxia and hypoxia in tumours — implications for treatment response. Br. J. Radiol. 87, 20130676 (2014).
Furusawa, Y. et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated 3He-, 12C- and 20Ne-ion beams. Radiat. Res. 154, 485–496 (2000).
Tinganelli, W. et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci. Rep. 5, 17016 (2015).
Horsman, M. R. et al. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 9, 674–687 (2012).
De Ruysscher, D., Haustermans, K. & Thorwarth, D. FDG and beyond. Recent Results Cancer Res. 198, 163–173 (2016).
Flynn, R. T., Bowen, S. R., Bentzen, S. M., Rockwell Mackie, T. & Jeraj, R. Intensity-modulated x-ray (IMXT) versus proton (IMPT) therapy for theragnostic hypoxia-based dose painting. Phys. Med. Biol. 53, 4153–4167 (2008).
Bassler, N. et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol. 53, 25–32 (2014).
Formenti, S. & Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 10, 718–726 (2009).
Weichselbaum, R. R., Liang, H., Deng, L. & Fu, Y.-X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. http://dx.doi.org/10.1038/nrclinonc.2016.211 (2017).
Shaked, Y. Balancing efficacy of and host immune responses to cancer therapy: the yin and yang effects. Nat. Rev. Clin. Oncol. 13, 611–626 (2016).
Durante, M., Brenner, D. J. & Formenti, S. C. Does heavy ion therapy work through the immune system? Int. J. Radiat. Oncol. Biol. Phys. 96, 934–936 (2016).
Yin, X. et al. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes. Sci. Rep. 5, 11373 (2015).
Shimokawa, T., Ma, L., Ando, K., Sato, K. & Imai, T. The future of combining carbon-ion radiotherapy with immunotherapy: evidence and progress in mouse models. Int. J. Part. Ther. 3, 61–70 (2016).
Yovino, S., Kleinberg, L., Grossman, S. A., Narayanan, M. & Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 31, 140–144 (2013).
Durante, M. et al. X-rays versus carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int. J. Radiat. Oncol. Biol. Phys. 47, 793–798 (2000).
Pignalosa, D. et al. Chromosome inversions in lymphocytes of prostate cancer patients treated with X-rays and carbon ions. Radiother. Oncol. 109, 256–261 (2013).
Takagi, M. et al. Treatment outcomes of particle radiotherapy using protons or carbon ions as a single-modality therapy for adenoid cystic carcinoma of the head and neck. Radiother. Oncol. 113, 364–370 (2014).
Krämer, M. et al. Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med. Phys. 43, 1995–2004 (2016).
Rovituso, M. et al. Fragmentation of 120 and 200 MeV u−14 He ions in water and PMMA targets. Phys. Med. Biol. 62, 1310–1326 (2017).
Tommasino, F., Scifoni, E. & Durante, M. New ions for therapy. Int. J. Part. Ther. 2, 428–438 (2015).
Knäusl, B., Fuchs, H., Dieckmann, K. & Georg, D. Can particle beam therapy be improved using helium ions? — a planning study focusing on pediatric patients. Acta Oncol. 55, 751–759 (2016).
Scifoni, E. et al. Including oxygen enhancement ratio in ion beam treatment planning: model implementation and experimental verification. Phys. Med. Biol. 58, 3871–3895 (2013).
Kurz, C., Mairani, A. & Parodi, K. First experimental-based characterization of oxygen ion beam depth dose distributions at the Heidelberg Ion-Beam Therapy Center. Phys. Med. Biol. 57, 5017–5034 (2012).
Hall, J. A., Salgado, R., Lively, T., Sweep, F. & Schuh, A. A risk-management approach for effective integration of biomarkers in clinical trials: perspectives of an NCI, NCRI, and EORTC working group. Lancet Oncol. 15, e184–e193 (2014).
O'Connor, J. P. B. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 14, 169–186 (2017).
Qutub, M. A. Z., Klein, S. B. & Buchsbaum, J. C. Rapid RBE-weighted proton radiation dosimetry risk assessment. Technol. Cancer Res. Treat. 15, NP1–NP7 (2016).
Tommasino, F. & Durante, M. Proton radiobiology. Cancers (Basel). 7, 353–381 (2015).
Pignalosa, D. & Durante, M. Overcoming resistance of cancer stem cells. Lancet Oncol. 13, e187–e188 (2012).
Laine, A. et al. International symposium on ion therapy: planning the first hospital-based heavy ion therapy center in the United States. Int. J. Part. Ther. 2, 468–471 (2016).
Roach, M. et al. New clinical and research programs in particle beam radiation therapy: the University of California San Francisco perspective. Int. J. Part. Ther. 2, 471–473 (2016).
Patel, S. et al. Recommendations for the referral of patients for proton-beam therapy, an Alberta Health Services report: a model for Canada? Curr. Oncol. 21, 251–262 (2014).
Shah, A., Ricci, K. I. & Efstathiou, J. A. Beyond a moonshot: insurance coverage for proton therapy. Lancet Oncol. 17, 559–561 (2016).
Acknowledgements
We thank Kristjan Anderle, Annabelle Becker, Anthony Magliari, and Emanuele Scifoni for their assistance with figures. We are also grateful to Noah Chan Choi and David Grosshans for providing useful information on the clinical trials on lung.
Author information
Authors and Affiliations
Contributions
M.D. researched data for the article. M.D., R.O. and J.S.L. wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.S.L. declares an association with ProCure Proton Therapy. R.O. and M.D. declare no competing interests.
Related links
FURTHER INFORMATION
Glossary
- Bragg peak
-
Bragg peak is the peak in the curve representing the energy loss of charged particles plotted against the depth in the material. The peak occurs immediately before the particle stops. If beams of different energies are used to irradiate a target volume, the narrow Bragg peak will be enlarged to cover the whole volume (spread-out-Bragg-peak, SOBP).
- Passive scattering
-
Passive scattering is a dose-delivery system in particle therapy in which a broad monoenergetic beam is used to treat a tumour. The energy variation is obtained with compensating filters of different depths and the shape is controlled with patient-specific collimators.
- Pencil-beam scanning
-
Pencil-beam scanning (PBS) is a dose-delivery system in particle therapy in which the beam is concentrated in spots of a few millimeters of diameter, and scanned through a 2D tumour slice. By changing the energy, a new slice can be scanned.
- Intensity-modulated proton therapy
-
Intensity-modulated proton therapy (IMPT) is a dose-delivery system in proton therapy in which the intensity of each pencil beam is modified to achieve a better target coverage. Intensity modulation is also used in X-ray-therapy.
- Tumour-control probability
-
Tumour-control probability (TCP) is the probability to sterilize a localized tumour volume. The tumour-control probability is generally higher than that of survival, which is affected by the occurrence of distant metastasis.
- Relative biological effectiveness
-
Relative biological effectiveness (RBE) is the ratio of the dose of reference radiation (generally X-rays or γ-rays) to test radiation (for example, protons or heavy ions) that produces the same biological effect. Higher RBE values are associated with increased effectiveness.
- Physical dose distribution
-
Physical dose distribution is the pattern of energy deposition in the body after exposure to ionizing radiation.
- Stereotactic body radiation therapy
-
Stereotactic body radiation therapy (SBRT) is a type of radiotherapy in which special equipment is used to position a patient and precisely deliver the dose to an extracranial tumour. The method requires high-quality imaging and can be delivered in much fewer fractions than conventional radiotherapy.
- Target volume
-
Target volume is the volume to be irradiated in radiotherapy. Several target volumes are considered in treatment planning. The gross tumour volume (GTV) corresponds to the visible tumour; the clinical target volume (CTV) includes the visible tumour and subclinical malignant extensions (GTV + margin); the internal target volume (ITV) includes the region where the CTV is moving (for example, during breathing); and the planning target volume (PTV) includes additional margins required to compensate for set-up uncertainties.
- Water-equivalent path length
-
Water-equivalent path length (WEPL) is the distance in centimeters that a proton beam in a nonhomogeneous tissue (with different densities) would have traversed in water.
- Dose painting
-
Dose painting is a heterogenous dose-delivery method used to increase the dose delivered to resistant tumour subvolumes.
- Dose halo
-
Dose halo is the peripheral dose around the pencil beam caused by scattering of the primary particles.
Rights and permissions
About this article
Cite this article
Durante, M., Orecchia, R. & Loeffler, J. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol 14, 483–495 (2017). https://doi.org/10.1038/nrclinonc.2017.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.30
This article is cited by
-
Comparison of sexual function after robot-assisted radical prostatectomy and carbon-ion radiotherapy for Japanese prostate cancer patients using propensity score matching
BMC Cancer (2024)
-
Mechanistic model of radiotherapy-induced lung fibrosis using coupled 3D agent-based and Monte Carlo simulations
Communications Medicine (2024)
-
Real-time single-proton counting with transmissive perovskite nanocrystal scintillators
Nature Materials (2024)
-
Positron emission tomography: its 65 years and beyond
La Rivista del Nuovo Cimento (2024)
-
Proton beam radiotherapy for choroidal and ciliary body melanoma in the UK—national audit of referral patterns of 1084 cases
Eye (2023)