Key Points
-
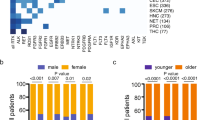
Oncogenic gene fusions are common in patients with solid tumours and occur across a wide spectrum of tumour types
-
Common methods of fusion detection used in the clinic include break-apart fluorescence in situ hybridization (FISH), immunohistochemistry, and next-generation sequencing
-
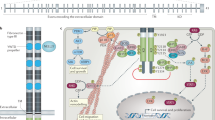
Gene fusions frequently involve tyrosine kinases and can cause constitutive kinase activation, augmentation of downstream signalling, and tumour proliferation
-
Targeted therapies are remarkably effective and are approved for patients with fusions involving ALK, ROS1, and PDGFB, and show promise in tumours harbouring fusions in RET, NTRK1/2/3, FGFR1/2/3, and BRAF/CRAF
-
Despite the success of tyrosine-kinase inhibitors (TKIs) in treating tumours with kinase fusions, resistance often develops owing to secondary mutations that disrupt drug binding
-
Treatment with later-generation TKIs can overcome acquired resistance and lead to improved outcomes for patients
Abstract
Structural gene rearrangements resulting in gene fusions are frequent events in solid tumours. The identification of certain activating fusions can aid in the diagnosis and effective treatment of patients with tumours harbouring these alterations. Advances in the techniques used to identify fusions have enabled physicians to detect these alterations in the clinic. Targeted therapies directed at constitutively activated oncogenic tyrosine kinases have proven remarkably effective against cancers with fusions involving ALK, ROS1, or PDGFB, and the efficacy of this approach continues to be explored in malignancies with RET, NTRK1/2/3, FGFR1/2/3, and BRAF/CRAF fusions. Nevertheless, prolonged treatment with such tyrosine-kinase inhibitors (TKIs) leads to the development of acquired resistance to therapy. This resistance can be mediated by mutations that alter drug binding, or by the activation of bypass pathways. Second-generation and third-generation TKIs have been developed to overcome resistance, and have variable levels of activity against tumours harbouring individual mutations that confer resistance to first-generation TKIs. The rational sequential administration of different inhibitors is emerging as a new treatment paradigm for patients with tumours that retain continued dependency on the downstream kinase of interest.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stransky, N., Cerami, E., Schalm, S., Kim, J. L. & Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 5, 4846 (2014).
Mitelman, F., Johansson, B. & Mertens, F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer 7, 233–245 (2007).
Nowell, P. & Hungerford, D. Minute chromosome in human chronic granulocytic leukemia. Science 132, 1497–1497 (1960).
Rowley, J. D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293 (1973).
Mertens, F., Johansson, B., Fioretos, T. & Mitelman, F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer 15, 371–381 (2015).
Mark, J., Dahlenfors, R., Ekedahl, C. & Stenman, G. The mixed salivary gland tumor — a normally benign human neoplasm frequently showing specific chromosomal abnormalities. Cancer Genet. Cytogenet. 2, 231–241 (1980).
Kas, K. et al. Promoter swapping between the genes for a novel zinc finger protein and β-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat. Genet. 15, 170–174 (1997).
Aurias, A., Rimbaut, C., Buffe, D., Dubousset, J. & Mazabraud, A. Chromosomal translocations in Ewing's sarcoma. N. Engl. J. Med. 309, 496–497 (1983).
Turc-Carel, C., Philip, I., Berger, M., Philip, T. & Lenoir, G. Chromosomal translocations in Ewing's sarcoma. N. Engl. J. Med. 309, 497–498 (1983).
Delattre, O. et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359, 162–165 (1992).
de Jong, B., Molenaar, I. M., Leeuw, J. A., Idenberg, V. J. & Oosterhuis, J. W. Cytogenetics of a renal adenocarcinoma in a 2-year-old child. Cancer Genet. Cytogenet. 21, 165–169 (1986).
Stenman, G., Sandros, J., Dahlenfors, R., Juberg-Ode, M. & Mark, J. 6q- and loss of the Y chromosome — two common deviations in malignant human salivary gland tumors. Cancer Genet. Cytogenet. 22, 283–293 (1986).
Sidhar, S. K. et al. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum. Mol. Genet. 5, 1333–1338 (1996).
Persson, M. et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl Acad. Sci. USA 106, 18740–18744 (2009).
Langer-Safer, P. R., Levine, M. & Ward, D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc. Natl Acad. Sci. USA 79, 4381–4385 (1982).
Bongarzone, I. et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene 4, 1457–1462 (1989).
Greco, A., Mariani, C., Miranda, C., Pagliardini, S. & Pierotti, M. A. Characterization of the NTRK1 genomic region involved in chromosomal rearrangements generating TRK oncogenes. Genomics 18, 397–400 (1993).
Grieco, M. et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60, 557–563 (1990).
Soda, M. et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007).
Fusco, A. et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 328, 170–172 (1987).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Meyerson, M., Gabriel, S. & Getz, G. Advances in understanding cancer genomes through second-generation sequencing. Nat. Rev. Genet. 11, 685–696 (2010).
Archer. Archer FusionPlex. ArcherDX http://archerdx.com/fusionplex-assays/ (2017).
Mitelman, F., Johansson, B. & Mertens, F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. https://ulib.iupui.edu/databases/mitelman-database-chromosome-aberrations-and-gene-fusions-cancer (2014).
The Jackson Laboratory. TCGA fusion gene data portal: landscape of cancer-associated fusions using the pipeline for RNA sequencing data analysis. The Jackson Laboratory http://54.84.12.177/PanCanFusV2/ (2017).
Bourgon, R. et al. High-throughput detection of clinically relevant mutations in archived tumor samples by multiplexed PCR and next-generation sequencing. Clin. Cancer Res. 20, 2080–2091 (2014).
Lou, D. I. et al. High-throughput DNA sequencing errors are reduced by orders of magnitude using circle sequencing. Proc. Natl Acad. Sci. USA 110, 19872–19877 (2013).
Hedegaard, J. et al. Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS ONE 9, e98187 (2014).
Ozsolak, F. & Milos, P. M. RNA sequencing: advances, challenges and opportunities. Nat. Rev. Genet. 12, 87–98 (2011).
Zhang, Y. et al. Chimeric transcript generated by cis-splicing of adjacent genes regulates prostate cancer cell proliferation. Cancer Discov. 2, 598–607 (2012).
Li, H., Wang, J., Mor, G. & Sklar, J. A neoplastic gene fusion mimics trans-splicing of RNAs in normal human cells. Science 321, 1357–1361 (2008).
Cheng, D. T. et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Pailler, E. et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 31, 2273–2281 (2013).
Food And Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). FDA https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431 (2017).
Bubendorf, L. et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 469, 489–503 (2016).
Frampton, G. M. et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 31, 1023–1031 (2013).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Cui, S. et al. Use of capture-based next-generation sequencing to detect ALK fusion in plasma cell-free DNA of patients with non-small-cell lung cancer. Oncotarget 8, 2771–2780 (2017).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Yoshihara, K. et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 34, 4845–4854 (2015).
Chmielecki, J. et al. Targeted next-generation sequencing of DNA regions proximal to a conserved GXGXXG signaling motif enables systematic discovery of tyrosine kinase fusions in cancer. Nucleic Acids Res. 38, 6985–6996 (2010).
Wang, R. et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 30, 4352–4359 (2012).
Hutchinson, K. E. et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin. Cancer Res. 19, 6696–6702 (2013).
Wu, Y.-M. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013).
Parker, B. C. & Zhang, W. Fusion genes in solid tumors: an emerging target for cancer diagnosis and treatment. Chin. J. Cancer 32, 594–603 (2013).
Majewski, I. J. et al. Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centred RNA sequencing. J. Pathol. 230, 270–276 (2013).
Shaw, A. T., Hsu, P. P., Awad, M. M. & Engelman, J. A. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer 13, 772–787 (2013).
Ciampi, R. et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115, 94–101 (2005).
May, W. A. et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc. Natl Acad. Sci. USA 90, 5752–5756 (1993).
Owen, L. A., Kowalewski, A. A. & Lessnick, S. L. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing's sarcoma. PLoS ONE 3, e1965 (2008).
Selvanathan, S. P. et al. Oncogenic fusion protein EWS-FLI1 is a network hub that regulates alternative splicing. Proc. Natl Acad. Sci. USA 112, E1307–E1316 (2015).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Sirvent, N., Maire, G. & Pedeutour, F. Genetics of dermatofibrosarcoma protuberans family of tumors: from ring chromosomes to tyrosine kinase inhibitor treatment. Genes Chromosomes Cancer 37, 1–19 (2003).
Kumar-Sinha, C., Kalyana-Sundaram, S. & Chinnaiyan, A. M. Landscape of gene fusions in epithelial cancers: seq and ye shall find. Genome Med. 7, 129 (2015).
French, C. A. Demystified molecular pathology of NUT midline carcinomas. J. Clin. Pathol. 63, 492–496 (2010).
Tognon, C. et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2, 367–376 (2002).
Ito, Y. et al. Mammary analogue secretory carcinoma of salivary glands: a clinicopathologic and molecular study including 2 cases harboring ETV6-X fusion. Am. J. Surg. Pathol. 39, 602–610 (2015).
Hughes, T. et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 108, 28–37 (2006).
Drilon, A. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. http://dx.doi.org/10.1158/2159-8290.CD-17-0507 (2017).
Roskoski, R. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res. 103, 26–48 (2016).
Singh, J., Petter, R. C., Baillie, T. A. & Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 10, 307–317 (2011).
Drilon, A. et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. 27, 920–926 (2016).
Doebele, R. C. et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin. Cancer Res. 18, 1472–1482 (2012).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl Med. 4, 120ra17 (2012).
Gainor, J. F. et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 6, 1118–1133 (2016).
Sasaki, T. et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 71, 6051–6060 (2011).
Toyokawa, G. et al. Identification of a novel ALK G1123S mutation in a patient with ALK-rearranged non-small-cell lung cancer exhibiting resistance to ceritinib. J. Thorac. Oncol. 10, e55–e57 (2015).
Katayama, R. et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin. Cancer Res. 20, 5686–5696 (2014).
Shaw, A. T. et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N. Engl. J. Med. 374, 54–61 (2016).
Ou, S.-H. et al. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer 91, 70–72 (2016).
Sakamoto, H. et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 19, 679–690 (2011).
Kodityal, S. et al. A novel acquired ALK F1245C mutation confers resistance to crizotinib in ALK-positive NSCLC but is sensitive to ceritinib. Lung Cancer 92, 19–21 (2016).
Ceccon, M. et al. Treatment efficacy and resistance mechanisms using the second-generation ALK inhibitor AP26113 in human NPM-ALK–positive anaplastic large cell loymphoma. Mol. Cancer Res. 13, 775–783 (2015).
Zou, H. Y. et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 28, 70–81 (2015).
Song, A. et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non-small cell lung cancer. Clin. Cancer Res. 21, 2379–2387 (2015).
Davare, M. A. et al. Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc. Natl Acad. Sci. USA 112, E5381–E5390 (2015).
Zou, H. Y. et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl Acad. Sci. USA 112, 3493–3498 (2015).
Drilon, A. et al. A novel crizotinib-resistant solvent-front mutation responsive to cabozantinib therapy in a patient with ROS1-rearranged lung cancer. Clin. Cancer Res. 22, 2351–2358 (2016).
Katayama, R. et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion–positive cancer. Clin. Cancer Res. 21, 166–174 (2015).
Mologni, L., Redaelli, S., Morandi, A., Plaza-Menacho, I. & Gambacorti-Passerini, C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol. Cell. Endocrinol. 377, 1–6 (2013).
Yoh, K. et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir. Med. 5, 42–50 (2017).
Drilon, A. et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 17, 1653–1660 (2016).
Yakes, F. M. et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10, 2298–2308 (2011).
Vaishnavi, A. et al. Oncogenic and drug sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 19, 1469–1472 (2013).
Menichincheri, M. et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (pan-TRKs) inhibitor. J. Med. Chem. 59, 3392–3408 (2016).
Estrada-Bernal, A., Le, A. T., Tuch, B., Kutateladze, T. G. & Doebele, R. C. Identification of TRKA and TRKB kinase domain mutations that induce resistance to a pan-TRK inhibitor [abstract]. Cancer Res. 76, LB-118 (2016).
Wang, X. et al. Development of peptidomimetic inhibitors of the ERG gene fusion product in prostate cancer. Cancer Cell 31, 532–548.e7 (2017).
Adamo, P. & Ladomery, M. R. The oncogene ERG: a key factor in prostate cancer. Oncogene 35, 403–414 (2016).
Takeuchi, K. et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 18, 378–381 (2012).
Wong, D. W.-S. et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115, 1723–1733 (2009).
Pan, Y. et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 84, 121–126 (2014).
Lin, E. et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol. Cancer Res. 7, 1466–1476 (2009).
Wiesner, T. et al. Kinase fusions are frequent in Spitz tumors and spitzoid melanomas. Nat. Commun. 5, 3116 (2014).
Drilon, A. et al. Safety and antitumor activity of the multi-targeted pan-TRK, ROS1, and ALK inhibitor entrectinib (RXDX-101): combined results from two phase 1 trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 4, 400–409 (2017).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Shaw, A. T. et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 370, 1189–1197 (2014).
Scagliotti, G., Crino, L., Liu, G. & Gridelli, C. Ceritinib versus chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with CT and crizotinib (CRZ): results from the confirmatory phase 3 ASCEND-5 study [abstract]. Ann. Oncol. 27, LBA42_PR (2016).
Hida, T. et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390, 29–39 (2017).
Gadgeel, S. M. et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 34, 4079–4085 (2016).
Wolf, J. et al. ALUR: a phase 3 study of alectinib versus chemotherapy in previously treated ALK+ non-small cell lung cancer (NSCLC) [abstract]. Ann. Oncol. 27, a1290TiP (2016).
Kim, D.-W. et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J. Clin. Oncol. 35, 2490–2498 (2017).
Soria, J.-C. et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389, 917–929 (2017).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1704795 (2017).
Solomon, B. J. et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J. Clin. Oncol. 34, 9009–9009 (2016).
Yamaguchi, N. et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer 83, 37–43 (2014).
Lovly, C. M. et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat. Med. 20, 1027–1034 (2014).
Mossé, Y. P. et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 14, 472–480 (2013).
Davies, K. D. & Doebele, R. C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 19, 4040–4045 (2013).
Shaw, A. T. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 371, 1963–1971 (2014).
Awad, M. M. et al. Acquired resistance to crizotinib from a mutation in CD74–ROS1. N. Engl. J. Med. 368, 2395–2401 (2013).
Davies, K. D. et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS ONE 8, e82236 (2013).
Lim, S. et al. Ceritinib in ROS1-rearranged non-small-cell lung cancer: a Korean nationwide phase II study. Ann. Oncol. 27, 415–454 (2016).
Gettinger, S. N. et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 17, 1683–1696 (2016).
Mendenhall, W. M., Zlotecki, R. A. & Scarborough, M. T. Dermatofibrosarcoma protuberans. Cancer 101, 2503–2508 (2004).
Rutkowski, P. et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J. Clin. Oncol. 28, 1772–1779 (2010).
Lee, S.-H. et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann. Oncol. 28, 292–297 (2016).
Velcheti, V. et al. Phase 2 study of lenvatinib (LN) in patients (pts) with RET fusion-positive adenocarcinoma of the lung [abstract]. Ann. Oncol. 27, a1204PD (2016).
Gautschi, O. et al. Targeting RET in patients with RET-rearranged lung cancers: results from a global, multicenter RET registry. J. Clin. Oncol. 35, 1403–1410 (2016).
Martin-Zanca, D., Hughes, S. H. & Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319, 743–748 (1986).
Vaishnavi, A., Le, A. T. & Doebele, R. C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 5, 25–34 (2015).
Amatu, A., Sartore-Bianchi, A. & Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 1, e000023 (2016).
Hyman, D. M. et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers [abstract]. J. Clin. Oncol. 35, LBA2501 (2017).
Russo, M. et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 6, 36–44 (2016).
Nanda, N., Fennell, T., Brandhuber, B., Tuch, B. B. & Low, J. A. Identification of tropomyosin kinase receptor (TRK) point mutations in cancer [abstract]. J. Clin. Oncol. a1553 (2015).
Helsten, T. et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 22, 259–267 (2016).
Turner, N. & Grose, R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 10, 116–129 (2010).
Singh, D. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337, 1231–1235 (2012).
Wang, R. et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin. Cancer Res. 20, 4107–4114 (2014).
Tabernero, J. et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 33, 3401–3408 (2015).
Nogova, L. et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J. Clin. Oncol. 35, 157–165 (2016).
Mazzaferro, V. et al. ARQ 087, an oral pan- fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced and/or metastatic intrahepatic cholangiocarcinoma (iCCA) [abstract]. Ann. Oncol. 35, 4017 (2016).
Ross, J. S. et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int. J. Cancer 138, 881–890 (2016).
Poulikakos, P. I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 (2011).
Sievert, A. J. et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl Acad. Sci. USA 110, 5957–5962 (2013).
Jain, P. et al. Pediatric low-grade gliomas with CRAF gene fusions respond differentially to targeted therapeutics based on dimerization profiles [abstract LG-25]. Neuro. Oncol. 18, iii84 (2016).
Tap, W. D. et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N. Engl. J. Med. 373, 428–437 (2015).
Konduri, K. et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov. 6, 601–611 (2016).
Acknowledgements
A.M.S. gratefully acknowledges funding support from the National Institutes of Health (award T32-CA009207).
Author information
Authors and Affiliations
Contributions
A.M.S. and A.D. researched data for the article and made a substantial contribution to discussions of content. All authors made a substantial contribution to writing the manuscript and editing and/or reviewing the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
A.D. has received honoraria from and is an advisory board member for Ariad, AstraZeneca, Blueprint Medicines, Exelixis, Genentech/Roche, Ignyta and Loxo Oncology and has received research funding from Foundation Medicine. The other authors declare no competing interests.
Supplementary information
Supplementary Figure 1
Introduction of agents and novel detection methods for oncogenic fusions in solid tumours. (DOC 138 kb)
Rights and permissions
About this article
Cite this article
Schram, A., Chang, M., Jonsson, P. et al. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 14, 735–748 (2017). https://doi.org/10.1038/nrclinonc.2017.127
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.127
This article is cited by
-
In vitro and in vivo analyses on anti-NSCLC activity of apatinib: rediscovery of a new drug target V600E mutation
Cancer Cell International (2023)
-
Pan-tumor survey of ROS1 fusions detected by next-generation RNA and whole transcriptome sequencing
BMC Cancer (2023)
-
Rare molecular subtypes of lung cancer
Nature Reviews Clinical Oncology (2023)
-
Classification of IDH wild-type glioblastoma tumorspheres into low- and high-invasion groups based on their transcriptional program
British Journal of Cancer (2023)
-
Prevalence and clinico-genomic characteristics of patients with TRK fusion cancer in China
npj Precision Oncology (2023)