Abstract
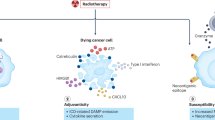
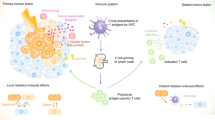
Conventional radiotherapy, in addition to its well-established tumoricidal effects, can also activate the host immune system. Radiation therapy modulates tumour phenotypes, enhances antigen presentation and tumour immunogenicity, increases production of cytokines and alters the tumour microenvironment, enabling destruction of the tumour by the immune system. Investigating the combination of radiotherapy with immunotherapeutic agents, which also promote the host antitumour immune response is, therefore, a logical progression. As the spectrum of clinical use of stereotactic radiotherapy continues to broaden, the question arose as to whether the ablative radiation doses used can also stimulate immune responses and, if so, whether we can amplify these effects by combining immunotherapy and stereotactic ablative radiotherapy (SABR). In this Perspectives article, we explore the preclinical and clinical evidence supporting activation of the immune system following SABR. We then examine studies that provide data on the effectiveness of combining these two techniques — immunotherapy and SABR — in an approach that we have termed 'ISABR'. Lastly, we provide general guiding principles for the development of future clinical trials to investigate the efficacy of ISABR in the hope of generating further interest in these exciting developments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Imaizumi, N., Monnier, Y., Hegi, M., Mirimanoff, R. O. & Ruegg, C. Radiotherapy suppresses angiogenesis in mice through TGF-βRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS ONE 5, e11084 (2010).
Kamrava, M., Bernstein, M. B., Camphausen, K. & Hodge, J. W. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol. Biosyst. 5, 1262–1270 (2009). This review examines preclinical and clinical data on the interaction between immunotherapy and radiation, and discusses the potential synergy between these two modalities and angiogenesis inhibitors.
Chen, Z. et al. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int. J. Cancer 93, 539–548 (2001).
Kotera, Y., Shimizu, K. & Mule, J. J. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 61, 8105–8109 (2001).
Melcher, A., Gough, M., Todryk, S. & Vile, R. Apoptosis or necrosis for tumor immunotherapy: what's in a name? J. Mol. Med. (Berl.) 77, 824–833 (1999).
Yu, P., Rowley, D. A., Fu, Y. X. & Schreiber, H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr. Opin. Immunol. 18, 226–231 (2006).
Ma, Y., Aymeric, L., Locher, C., Kroemer, G. & Zitvogel, L. The dendritic cell-tumor cross-talk in cancer. Curr. Opin. Immunol. 23, 146–152 (2011).
McBride, W. H. et al. A sense of danger from radiation. Radiat. Res. 162, 1–19 (2004).
Demaria, S., Bhardwaj, N., McBride, W. H. & Formenti, S. C. Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 63, 655–666 (2005).
Friedman, E. J. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr. Pharm. Des. 8, 1765–1780 (2002).
Kwilas, A. R., Donahue, R. N., Bernstein, M. B. & Hodge, J. W. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front. Oncol. 2, 104 (2012).
Gameiro, S. R. et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 5, 403–416 (2014). This study provided evidence that radiation induces a continuum of immunogenic alterations of tumour biology, from immunogenic modulation to immunogenic cell death.
Hodge, J. W., Ardiani, A., Farsaci, B., Kwilas, A. R. & Gameiro, S. R. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin. Oncol. 39, 323–339 (2012).
Gameiro, S. R., Caballero, J. A., Higgins, J. P., Apelian, D. & Hodge, J. W. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol. Immunother. 60, 1227–1242 (2011).
Hodge, J. W. et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int. J. Cancer 133, 624–636 (2013).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006). This study showed that directed radiotherapy can improve the efficacy of tumor immunotherapy and result in successful eradication of murine colon adenocarcinoma.
Chang, J. Y. et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer 119, 3402–3410 (2013).
Palma, D. A. et al. The oligometastatic state — separating truth from wishful thinking. Nat. Rev. Clin. Oncol. 11, 549–557 (2014).
Timmerman, R. D., Herman, J. & Cho, L. C. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J. Clin. Oncol. 32, 2847–2854 (2014).
Bernstein, M. B. et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother. Radiopharm. 29, 153–161 (2014).
Schaue, D., Ratikan, J. A., Iwamoto, K. S. & McBride, W. H. Maximizing tumor immunity with fractionated radiation. Int. J. Radi. Oncol. Biol. Phys. 83, 1306–1310 (2012).
Formenti, S. C. & Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radi. Oncol. Biol. Phys. 84, 879–880 (2012). This paper summarizes important evidence that local radiotherapy and immunotherapy can successfully synergize and produce therapeutically effective anti-tumour immune responses, even in metastatic cancer.
Zhang, H. et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PLoS ONE 7, e38111 (2012).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 127, 759–767 (2010).
Aryankalayil, M. J. et al. Defining molecular signature of pro-immunogenic radiotherapy targets in human prostate cancer cells. Radiat. Res. 182, 139–148 (2014).
Said, E. A. et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 (2010).
Egen, J. G., Kuhns, M. S. & Allison, J. P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3, 611–618 (2002).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Verbrugge, I. et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72, 3163–3174 (2012).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Zegers, C. M. et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin. Cancer Res. 21, 1151–1160 (2015).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012). This paper details the abscopal effect, a phenomenon in which local radiotherapy is associated with regression of metastatic cancer at a distance from the irradiate site.
Hodge, J. W., Sharp, H. J. & Gameiro, S. R. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother. Radiopharm. 27, 12–22 (2012).
Hiniker, S. M. et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 5, 404–407 (2012).
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A. & Formenti, S. C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 1, 365–372 (2013).
Silk, A. W., Bassetti, M. F., West, B. T., Tsien, C. I. & Lao, C. D. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2, 899–906 (2013).
Stamell, E. F., Wolchok, J. D., Gnjatic, S., Lee, N. Y. & Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013).
Karbach, J. et al. Long-term complete remission following radiosurgery and immunotherapy in a melanoma patient with brain metastasis: immunologic correlates. Cancer Immunol. Res. 2, 404–409 (2014).
Kiess, A. P. et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 92, 368–375 (2015).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
Seung, S. K. et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2 — tumor and immunological responses. Sci. Transl. Med. 4, 137ra174 (2012).
US National Library of Science. ClinicalTrials.gov [online] (2014).
US National Library of Science. ClinicalTrials.gov [online] (2014).
US National Library of Science. ClinicalTrials.gov [online] (2014)
Crittenden, M. et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin. Radiat. Oncol. 25, 54–64 (2015).
Chang, J. Y. et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat. Oncol. 7, 152 (2012).
Timmerman, R. et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303, 1070–1076 (2010).
Senthi, S., Lagerwaard, F. J., Haasbeek, C. J., Slotman, B. J. & Senan, S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 13, 802–809 (2012).
Timmerman, R. et al. RTOG 0618: stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J. Clin. Oncol. 31, S7523 (2013).
Chang, J. Y. et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 16, 630–637 (2015). An important study showing SABR as an option for therapy in operable stage I non-small cell lung cancer.
Shirvani, S. M. et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 149, 1244–1253 (2014).
Vansteenkiste, J. F. et al. MAGRIT, a double-blind, randomized, placebo-controlled Phase III study to assess the efficacy of the recMAGE-A3 + AS15 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small cell lung cancer (NSCLC). Presented at the European Society for Medical Oncology, Madrid Spain (2014).
US National Library of Science. ClinicalTrials.gov [online] (2015).
Kalbasi, A., June, C. H., Haas, N. & Vapiwala, N. Radiation and immunotherapy: a synergistic combination. J. Clin. Invest. 123, 2756–2763 (2013).
Young, K., Cottam, B., Baird, J. R., Gough, M. J. & Crittenden, M. Ideal timing of immunotherapy with radiation in murine tumor models. Int. J. Radiat. Oncol. 90, S58 (2014).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Henn, B. M., Botigue, L. R., Bustamante, C. D., Clark, A. G. & Gravel, S. Estimating the mutation load in human genomes. Nat. Rev. Genet. 16, 333–343 (2015).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Science Transl. Med. 6, 238ra270 (2014).
Kwek, S. S. et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J. Immunol. 189, 3759–3766 (2012).
Disis, M. L. et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol. Immunother. 63, 101–109 (2014).
Gulley, J. L. et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol. Res. 2, 133–141 (2014).
Rosenberg, S. A. et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 175, 6169–6176 (2005).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). This review discusses the promising approach of utilizing immune checkpoint blockade to enhance antitumour immunity with the potential to produce durable clinical responses.
US National Library of Science. ClinicalTrials.gov [online] (2015).
US National Library of Science. ClinicalTrials.gov [online] (2013).
US National Library of Science. ClinicalTrials.gov [online] (2015).
US National Library of Science. ClinicalTrials.gov [online] (2015).
US National Library of Science. ClinicalTrials.gov [online] (2015).
US National Library of Science. ClinicalTrials.gov [online] (2015).
Bolli, M. et al. Tissue microarray evaluation of Melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann. Surg. 236, 785–793; discussion 793 (2002).
Jungbluth, A. A. et al. Expression of MAGE-antigens in normal tissues and cancer. Int. J. Cancer 85, 460–465 (2000).
Author information
Authors and Affiliations
Contributions
All authors researched data for this manuscript, made a substantial contribution to discussions of content, wrote the manuscript and reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bernstein, M., Krishnan, S., Hodge, J. et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach?. Nat Rev Clin Oncol 13, 516–524 (2016). https://doi.org/10.1038/nrclinonc.2016.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.30
This article is cited by
-
Hypofractionated radiotherapy for refractory or relapsed aggressive B-cell lymphoma in the rituximab era
BMC Cancer (2024)
-
Characterization and clinical verification of immune-related genes in hepatocellular carcinoma to aid prognosis evaluation and immunotherapy
BMC Cancer (2023)
-
Radiotherapy plus anti-PD1 versus radiotherapy for hepatic toxicity in patients with hepatocellular carcinoma
Radiation Oncology (2023)
-
PET-based radiomics visualizes tumor-infiltrating CD8 T cell exhaustion to optimize radiotherapy/immunotherapy combination in mouse models of lung cancer
Biomarker Research (2023)
-
Initial analysis of the synergy of programmed cell death-1 (PD-1) inhibitor and concurrent chemoradiotherapy treatment for recurrent/metastatic head and neck squamous cell carcinoma patients
Radiation Oncology (2023)