Key Points
-
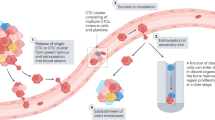
Distant metastasis remains a frequent cause of death, even after locoregional disease control is achieved using surgery, radiotherapy, and/or systemic therapy
-
Antitumour therapies can, under some circumstances, mobilize tumour cells into the peripheral circulation that might influence the risk of distant metastasis
-
Irradiation can enable tumour cells to acquire properties that facilitate their dissemination and the subsequent generation of metastases
-
Several mechanisms might explain a metastasis-promoting effect derived from surgical procedures, such as tissue disruption and leakage of blood containing tumour cells
-
A potential link between systemic therapies and metastasis has not been established, but the results of isolated studies indicate that this question needs to be addressed
-
The risk of distant failure from antitumour therapy can potentially be reduced if treatment-related factors capable of promoting metastasis are identified, and targeted therapeutically
Abstract
Despite progressive improvements in the management of patients with locoregionally confined, advanced-stage solid tumours, distant metastasis remains a very common — and usually fatal — mode of failure after attempted curative treatment. Surgery and radiotherapy are the primary curative modalities for these patients, often combined with each other and/or with chemotherapy. Distant metastasis occurring after treatment can arise from previously undetected micrometastases or, alternatively, from persistent locoregional disease. Another possibility is that treatment itself might sometimes cause or promote metastasis. Surgical interventions in patients with cancer, including biopsies, are commonly associated with increased concentrations of circulating tumour cells (CTCs). High CTC numbers are associated with an unfavourable prognosis in many cancers. Radiotherapy and systemic antitumour therapies might also mobilize CTCs. We review the preclinical and clinical data concerning cancer treatments, CTC mobilization and other factors that might promote metastasis. Contemporary treatment regimens represent the best available curative options for patients who might otherwise die from locally confined, advanced-stage cancers; however, if such treatments can promote metastasis, this process must be understood and addressed therapeutically to improve patient survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pantel, K. et al. Circulating epithelial cells in patients with benign colon diseases. Clin. Chem. 58, 936–940 (2012).
Sanger, N. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Weigelt, B. et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc. Natl Acad. Sci. USA 100, 15901–15905 (2003).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904 (2004).
Lucci, A. et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 13, 688–695 (2012).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Coumans, F. A., Siesling, S. & Terstappen, L. W. Detection of cancer before distant metastasis. BMC Cancer 13, 283 (2013).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Naxerova, K. & Jain, R. K. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat. Rev. Clin. Oncol. 12, 258–272 (2015).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Comen, E., Norton, L. & Massague, J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 8, 369–377 (2011).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Toss, A., Mu, Z., Fernandez, S. & Cristofanilli, M. CTC enumeration and characterization: moving toward personalized medicine. Ann. Transl Med. 2, 108 (2014).
Schwarzenbach, H. et al. Loss of heterozygosity at tumor suppressor genes detectable on fractionated circulating cell-free tumor DNA as indicator of breast cancer progression. Clin. Cancer Res. 18, 5719–5730 (2012).
Spindler, K. L., Pallisgaard, N., Andersen, R. F. & Jakobsen, A. Changes in mutational status during third-line treatment for metastatic colorectal cancer—results of consecutive measurement of cell free DNA, KRAS and BRAF in the plasma. Int. J. Cancer 135, 2215–2222 (2014).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Bonnomet, A. et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J. Mammary Gland Biol. Neoplasia 15, 261–273 (2010).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Lafrenie, R., Shaughnessy, S. G. & Orr, F. W. Cancer cell interactions with injured or activated endothelium. Cancer Metastasis Rev. 11, 377–388 (1992).
Friedl, P., Locker, J., Sahai, E. & Segall, J. E. Classifying collective cancer cell invasion. Nat. Cell Biol. 14, 777–783 (2012).
Rofstad, E. K., Galappathi, K. & Mathiesen, B. S. Tumor interstitial fluid pressure-a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia 16, 586–594 (2014).
Robinson, B. D. et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin. Cancer Res. 15, 2433–2441 (2009).
Roussos, E. T. et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci. 124, 2120–2131 (2011).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Hou, J. M. et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 178, 989–996 (2011).
Brandt, B. et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 56, 4556–4561 (1996).
Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Martin, O. A. et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 88, 395–403 (2014).
Pretlow, T. G. et al. Prostate cancer and other xenografts from cells in peripheral blood of patients. Cancer Res. 60, 4033–4036 (2000).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Hodgkinson, C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20, 897–903 (2014).
Yu, M. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Levina, V., Marrangoni, A. M., DeMarco, R., Gorelik, E. & Lokshin, A. E. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE 3, e3077 (2008).
Gomez-Casal, R. et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 12, 94 (2013).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573 (1889).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Hood, J. L., San, R. S. & Wickline, S. A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 (2011).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
von Essen, C. F. Radiation enhancement of metastasis: a review. Clin. Exp. Metastasis 9, 77–104 (1991).
Kaplan, H. S. & Murphy, E. D. The effect of local roentgen irradiation on the biological behavior of a transplantable mouse carcinoma; increased frequency of pulmonary metastasis. J. Natl Cancer Inst. 9, 407–413 (1949).
Sheldon, P. W. & Fowler, J. F. The effect of low-dose pre-operative X-irradiation of implanted mouse mammary carcinomas on local recurrence and metastasis. Br. J. Cancer 34, 401–407 (1976).
Camphausen, K. et al. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 61, 2207–2211 (2001).
Rofstad, E. K., Mathiesen, B. & Galappathi, K. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res. 64, 13–18 (2004).
Park, J. K. et al. Establishment of animal model for the analysis of cancer cell metastasis during radiotherapy. Radiat. Oncol. 7, 153 (2012).
Li, T. et al. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial–mesenchymal transition. Cancer Gene Ther. 18, 617–626 (2011).
Woods, G. M. et al. Radiation therapy may increase metastatic potential in alveolar rhabdomyosarcoma. Pediatr. Blood Cancer 62, 1550–1554 (2015).
Bernier, J., Hall, E. J. & Giaccia, A. Radiation oncology: a century of achievements. Nat. Rev. Cancer 4, 737–747 (2004).
Eriksson, D. & Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 31, 363–372 (2010).
Dewey, W. C., Ling, C. C. & Meyn, R. E. Radiation-induced apoptosis: relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 33, 781–796 (1995).
Palumbo, S. & Comincini, S. Autophagy and ionizing radiation in tumors: the “survive or not survive” dilemma. J. Cell. Physiol. 228, 1–8 (2013).
Brunner, T. B., Kunz-Schughart, L. A., Grosse-Gehling, P. & Baumann, M. Cancer stem cells as a predictive factor in radiotherapy. Semin. Radiat. Oncol. 22, 151–174 (2012).
Butof, R., Dubrovska, A. & Baumann, M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 108, 388–396 (2013).
Jackson, S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696 (2002).
Roy, K., Kodama, S., Suzuki, K. & Watanabe, M. Delayed cell death, giant cell formation and chromosome instability induced by X-irradiation in human embryo cells. J. Radiat. Res. 40, 311–322 (1999).
De Bacco, F. et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J. Natl Cancer Inst. 103, 645–661 (2011).
Zheng, Q. et al. X-ray radiation promotes the metastatic potential of tongue squamous cell carcinoma cells via modulation of biomechanical and cytoskeletal properties. Hum. Exp. Toxicol. 34, 894–903 (2015).
Cui, Y. H. et al. Radiation promotes invasiveness of non-small-cell lung cancer cells through granulocyte-colony-stimulating factor. Oncogene 34, 5372–5382 (2015).
Vilalta, M., Rafat, M., Giaccia, A. J. & Graves, E. E. Recruitment of circulating breast cancer cells is stimulated by radiotherapy. Cell Rep. 8, 402–409 (2014).
Gorski, D. H. et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 59, 3374–3378 (1999).
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441 (2004).
Chung, Y. L. et al. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin. Cancer Res. 12, 2706–2715 (2006).
Sofia Vala, I. et al. Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PLoS ONE 5, e11222 (2010).
Shen, C. J. et al. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC Cancer 14, 532 (2014).
Budach, W., Kammers, K., Boelke, E. & Matuschek, C. Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials. Radiat. Oncol. 8, 267 (2013).
Errico, A. Radiotherapy: a double-edged sword for NSCLC? Nat. Rev. Clin. Oncol. 11, 66 (2014).
Bonner, W. M. et al. γH2AX and cancer. Nat. Rev. Cancer 8, 957–967 (2008).
Ivashkevich, A., Redon, C. E., Nakamura, A. J., Martin, R. F. & Martin, O. A. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 327, 123–133 (2012).
Saunders, M. et al. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 350, 161–165 (1997).
Saunders, M. et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother. Oncol. 52, 137–148 (1999).
Dorsey, J. F. et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer 121, 139–149 (2015).
Lowes, L. E. et al. Circulating tumour cells in prostate cancer patients receiving salvage radiotherapy. Clin. Transl Oncol. 14, 150–156 (2012).
Haikerwal, S. J., Hagekyriakou, J., MacManus, M., Martin, O. A. & Haynes, N. M. Building immunity to cancer with radiation therapy. Cancer Lett. 368, 198–208 (2015).
Rivinius, R. et al. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des. Devel. Ther. 9, 93–102 (2015).
Engels, E. A. et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306, 1891–1901 (2011).
Andarawewa, K. L. et al. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor β-induced epithelial to mesenchymal transition. Cancer Res. 67, 8662–8670 (2007).
Yuan, W., Yuan, Y., Zhang, T. & Wu, S. Role of Bmi-1 in regulation of ionizing irradiation-induced epithelial-mesenchymal transition and migration of breast cancer cells. PLoS ONE 10, e0118799 (2015).
Kim, R. K. et al. Radiation promotes malignant phenotypes through SRC in breast cancer cells. Cancer Sci. 106, 78–85 (2015).
Qian, L. W. et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin. Cancer Res. 8, 1223–1227 (2002).
Wasserman, J., Blomgren, H., Rotstein, S., Petrini, B. & Hammarstrom, S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull. NY Acad. Med. 65, 36–44 (1989).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Zhang, B. et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 204, 49–55 (2007).
Chakraborty, M. et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 170, 6338–6347 (2003).
Hall, E. J. Radiobiology for the Radiologist (Medical Department, Harper and Row, 1973).
Pateras, I. S. et al. The DNA damage response and immune signaling alliance: is it good or bad? Nature decides when and where. Pharmacol. Ther. 154, 36–56 (2015).
Scheithauer, H., Belka, C., Lauber, K. & Gaipl, U. S. Immunological aspects of radiotherapy. Radiat. Oncol. 9, 185 (2014).
Brown, J. M. The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce, F. Cain Memorial Award lecture. Cancer Res. 59, 5863–5870 (1999).
Shultz, D. B., Diehn, M. & Loo, B. W. Jr. To SABR or not to SABR? Indications and contraindications for stereotactic ablative radiotherapy in the treatment of early-stage, oligometastatic, or oligoprogressive non-small cell lung cancer. Semin. Radiat. Oncol. 25, 78–86 (2015).
Bertolaccini, L., Terzi, A., Ricchetti, F. & Alongi, F. Surgery or stereotactic ablative radiation therapy: how will be treated operable patients with early stage not small cell lung cancer in the next future? Ann. Transl Med. 3, 25 (2015).
Cao, C. et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol. 16, e370–e371 (2015).
Zheng, X. et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 90, 603–611 (2014).
Rekers, N. H. et al. Stereotactic ablative body radiotherapy combined with immunotherapy: present status and future perspectives. Cancer Radiother. 18, 391–395 (2014).
Narayan, K., Fisher, R. J., Bernshaw, D., Shakher, R. & Hicks, R. J. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int. J. Gynecol. Cancer 19, 912–918 (2009).
Narayan, K., Khaw, P., Bernshaw, D., Mileshkin, L. & Kondalsamy-Chennakesavan, S. Prognostic significance of lymphovascular space invasion and nodal involvement in intermediate- and high-risk endometrial cancer patients treated with curative intent using surgery and adjuvant radiotherapy. Int. J. Gynecol. Cancer 22, 260–266 (2012).
Fidler, I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur. J. Cancer 9, 223–227 (1973).
Liotta, L. A., Saidel, M. G. & Kleinerman, J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 36, 889–894 (1976).
Ceelen, W., Pattyn, P. & Mareel, M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 89, 16–26 (2014).
Alagaratnam, T. T. & Ong, G. B. Wound implantation — a surgical hazard. Br. J. Surg. 64, 872–875 (1977).
Heaney, A. & Buggy, D. J. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br. J. Anaesthesia 109 (Suppl. 1), i17–i28 (2012).
Ben-Eliyahu, S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav. Immun. 17 (Suppl. 1), 27–36 (2003).
Buckley, A., McQuaid, S., Johnson, P. & Buggy, D. J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br. J. Anaesthesia 113 (Suppl. 1), i56–62 (2014).
Divatia, J. V. & Ambulkar, R. Anesthesia and cancer recurrence: what is the evidence? J. Anaesthesiol. Clin. Pharmacol. 30, 147–150 (2014).
Cata, J. P., Wang, H., Gottumukkala, V., Reuben, J. & Sessler, D. I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesthesia 110, 690–701 (2013).
Demicheli, R., Retsky, M. W., Hrushesky, W. J., Baum, M. & Gukas, I. D. The effects of surgery on tumor growth: a century of investigations. Ann. Oncol. 19, 1821–1828 (2008).
Horowitz, M., Neeman, E., Sharon, E. & Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 12, 213–226 (2015).
Holmgren, L., O'Reilly, M. S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1, 149–153 (1995).
Abramovitch, R., Marikovsky, M., Meir, G. & Neeman, M. Stimulation of tumour growth by wound-derived growth factors. Br. J. Cancer 79, 1392–1398 (1999).
Mathenge, E. G. et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 16, 950–960 (2014).
Moreno, J. G. et al. Transrectal ultrasound-guided biopsy causes hematogenous dissemination of prostate cells as determined by RT-PCR. Urology 49, 515–520 (1997).
Polascik, T. J. et al. Influence of sextant prostate needle biopsy or surgery on the detection and harvest of intact circulating prostate cancer cells. J. Urol. 162, 749–752 (1999).
Dyavanagoudar, S., Kale, A., Bhat, K. & Hallikerimath, S. Reverse transcriptase polymerase chain reaction study to evaluate dissemination of cancer cells into circulation after incision biopsy in oral squamous cell carcinoma. Indian J. Dental Res. 19, 315–319 (2008).
Kusukawa, J. et al. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 29, 303–307 (2000).
Zoubek, A. et al. Mobilization of tumour cells during biopsy in an infant with Ewing sarcoma. Eur. J. Pediatr. 155, 373–376 (1996).
Uchida, N., Suda, T., Inoue, T., Fujiwara, Y. & Ishiguro, K. Needle track dissemination of follicular thyroid carcinoma following fine-needle aspiration biopsy: report of a case. Surg. Today 37, 34–37 (2007).
Jones, O. M., Rees, M., John, T. G., Bygrave, S. & Plant, G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br. J. Surg. 92, 1165–1168 (2005).
Rodgers, M. S., Collinson, R., Desai, S., Stubbs, R. S. & McCall, J. L. Risk of dissemination with biopsy of colorectal liver metastases. Dis. Colon Rectum 46, 454–458; discussion 458–459 (2003).
Myrvang, H. Diagnosis: novel anti-seeding technology to prevent dissemination of tumor cells after a fine-needle aspiration biopsy. Nat. Rev. Clin. Oncol. 8, 64 (2011).
Wiksell, H. et al. Prevention of tumour cell dissemination in diagnostic needle procedures. Br. J. Cancer 103, 1706–1709 (2010).
Koch, M. et al. Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg. Endosc. 18, 587–591 (2004).
Koch, M. et al. Increased detection rate and potential prognostic impact of disseminated tumor cells in patients undergoing endorectal ultrasound for rectal cancer. Int. J. Colorectal Dis. 22, 359–365 (2007).
Peach, G., Kim, C., Zacharakis, E., Purkayastha, S. & Ziprin, P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br. J. Cancer 102, 1327–1334 (2010).
Rahbari, N. N. et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 138, 1714–1726 (2010).
Steinert, G., Scholch, S., Koch, M. & Weitz, J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbecks Arch. Surg. 397, 535–542 (2012).
Weitz, J. et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin. Cancer Res. 4, 343–348 (1998).
Rahbari, N. N. et al. Compartmental differences of circulating tumor cells in colorectal cancer. Ann. Surg. Oncol. 19, 2195–2202 (2012).
Koch, M. et al. Detection of disseminated tumor cells in liver biopsies of colorectal cancer patients is not associated with a worse prognosis. Ann. Surg. Oncol. 14, 810–817 (2007).
Wind, J. et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur. J. Surg. Oncol. 35, 942–950 (2009).
Kienle, P. et al. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann. Surg. 238, 324–330; discussion 330–321 (2003).
Rahbari, N. N. et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 20, 4169–4182 (2013).
Nesteruk, D. et al. Evaluation of prognostic significance of circulating tumor cells detection in rectal cancer patients treated with preoperative radiotherapy: prospectively collected material data. BioMed Res. Int. 2014, 712827 (2014).
Demicheli, R., Biganzoli, E., Boracchi, P., Greco, M. & Retsky, M. W. Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res. 10, R83 (2008).
Fortin, A., Larochelle, M., Laverdiere, J., Lavertu, S. & Tremblay, D. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J. Clin. Oncol. 17, 101–109 (1999).
Jatoi, I., Tsimelzon, A., Weiss, H., Clark, G. M. & Hilsenbeck, S. G. Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res. Treat. 89, 173–178 (2005).
Demicheli, R. et al. Breast cancer recurrence dynamics following adjuvant CMF is consistent with tumor dormancy and mastectomy-driven acceleration of the metastatic process. Ann. Oncol. 16, 1449–1457 (2005).
Hermansson, U., Konstantinov, I. E. & Aren, C. Tumor dissemination after video-assisted thoracic surgery: what does it mean? J. Thorac. Cardiovasc. Surg. 114, 300–302 (1997).
Yamashita, J. I., Kurusu, Y., Fujino, N., Saisyoji, T. & Ogawa, M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J. Thorac. Cardiovasc. Surg. 119, 899–905 (2000).
Hashimoto, M. et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact. Cardiovasc. Thorac. Surg. 18, 775–783 (2014).
Dong, Q. et al. Hematogenous dissemination of lung cancer cells during surgery: quantitative detection by flow cytometry and prognostic significance. Lung Cancer 37, 293–301 (2002).
Eschwege, P. et al. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 346, 1528–1530 (1995).
Daskalakis, M. et al. Assessment of the effect of surgery on the kinetics of circulating tumour cells in patients with operable breast cancer based on cytokeratin-19 mRNA detection. Eur. J. Surg. Oncol. 37, 404–410 (2011).
Engilbertsson, H. et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J. Urol. 193, 53–57 (2015).
Nakashima, S. et al. Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery 133, 162–169 (2003).
Kayalar, N. et al. Concomitant surgery for renal neoplasm with pulmonary tumor embolism. J. Thorac. Cardiovasc. Surg. 139, 320–325 (2010).
Podgrabinska, S. & Skobe, M. Role of lymphatic vasculature in regional and distant metastases. Microvasc. Res. 95, 46–52 (2014).
Liao, C. et al. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: a systematic review and meta-analysis. Cancer Biomarkers 14, 469–481 (2014).
Song, P. P., Zhang, W., Zhang, B., Liu, Q. & Du, J. Effects of different sequences of pulmonary artery and vein ligations during pulmonary lobectomy on blood micrometastasis of non-small cell lung cancer. Oncol. Lett. 5, 463–468 (2013).
Smerage, J. B. et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 32, 3483–3489 (2014).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Ebos, J. M. & Kerbel, R. S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8, 210–221 (2011).
Obenauf, A. C. et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 520, 368–372 (2015).
Itescu, S. et al. Intravenous pulse administration of cyclophosphamide is an effective and safe treatment for sensitized cardiac allograft recipients. Circulation 105, 1214–1219 (2002).
Schiavoni, G. et al. Cyclophosphamide induces type I interferon and augments the number of CD44hi T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood 95, 2024–2030 (2000).
Man, S., Zhang, Y., Gao, W., Yan, L. & Ma, C. Cyclophosphamide promotes pulmonary metastasis on mouse lung adenocarcinoma. Clin. Exp. Metastasis 25, 855–864 (2008).
Yamauchi, K. et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 68, 516–520 (2008).
Price, J. T. et al. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 65, 4929–4938 (2005).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Brugger, W. et al. Mobilization of peripheral blood progenitor cells by sequential administration of interleukin-3 and granulocyte-macrophage colony-stimulating factor following polychemotherapy with etoposide, ifosfamide, and cisplatin. Blood 79, 1193–1200 (1992).
Inhestern, J. et al. Prognostic role of circulating tumor cells during induction chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer. PLoS ONE 10, e0132901 (2015).
Mego, M. et al. Prognostic value of EMT-circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J. Cancer 3, 369–380 (2012).
Hamilton, B. K. et al. Long-term survival after high-dose chemotherapy with autologous hematopoietic cell transplantation in metastatic breast cancer. Hematol. Oncol. Stem Cell Ther. 8, 115–124 (2015).
Kuderer, N. M., Dale, D. C., Crawford, J. & Lyman, G. H. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J. Clin. Oncol. 25, 3158–3167 (2007).
Cao, Y. et al. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 74, 5091–5102 (2014).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010).
Debeljak, N., Solar, P. & Sytkowski, A. J. Erythropoietin and cancer: the unintended consequences of anemia correction. Front. Immunol. 5, 563 (2014).
Shenouda, G. et al. Long-term results of radiation therapy oncology group 9903: a randomized phase 3 trial to assess the effect of erythropoietin on local-regional control in anemic patients treated with radiation therapy for squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 91, 907–915 (2015).
NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 383, 1561–1571 (2014).
Liang, Y. & Wakelee, H. A. Adjuvant chemotherapy of completely resected early stage non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2, 403–410 (2013).
Mauri, D., Pavlidis, N. & Ioannidis, J. P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: ameta-analysis. J. Natl Cancer Inst. 97, 188–194 (2005).
Mac Manus, M. P. et al. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer 49, 95–108 (2005).
Acknowledgements
We are grateful to David Ball for fruitful discussions and his continuous support of our scientific initiatives, and to Bernhard Riedel for his critical reading of the manuscript. We thank Tim Akhurst for his assistance with Figure 3. O.A.M. and M.P.M. receive support from the Australian National Health and Medical Research Council (NHMRC) grant 1104139, and the Peter MacCallum Cancer Foundation grant 1218. R.L.A. receives fellowship support from the National Breast Cancer Foundation of Australia.
Author information
Authors and Affiliations
Contributions
O.A.M., R.L.A. and M.P.M. researched data for the article, and K.N. contributed to discussion of the article's content. All authors wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Martin, O., Anderson, R., Narayan, K. et al. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis?. Nat Rev Clin Oncol 14, 32–44 (2017). https://doi.org/10.1038/nrclinonc.2016.128
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.128
This article is cited by
-
Fluorouracil exacerbates alpha-crystallin B chain—mediated cell migration in triple-negative breast cancer cell lines
Scientific Reports (2023)
-
Influence of Perioperative Anesthesia on Cancer Recurrence: from Basic Science to Clinical Practice
Current Oncology Reports (2023)
-
An intravascular needle coated by ZnO nanoflowers for in vivo elimination of circulating tumor cells
Nano Research (2023)
-
AST·MLR index and operation injury condition are novel prognostic predictor for the prediction of survival in patients with colorectal cancer liver metastases undergoing surgical resection
BMC Cancer (2022)
-
Janus-Nanojet as an efficient asymmetric photothermal source
Scientific Reports (2022)